A New Benzothiazolthiazolidine Derivative, 11726172, Is Active In Vitro, In Vivo, and against Nonreplicating Cells of Mycobacterium tuberculosis
- PMID: 36377880
- PMCID: PMC9769805
- DOI: 10.1128/msphere.00369-22
A New Benzothiazolthiazolidine Derivative, 11726172, Is Active In Vitro, In Vivo, and against Nonreplicating Cells of Mycobacterium tuberculosis
Abstract
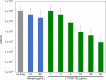
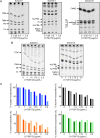
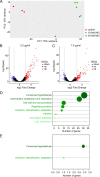
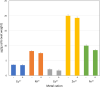
Tuberculosis (TB) still poses a global menace as one of the deadliest infectious diseases. A quarter of the human population is indeed latently infected with Mycobacterium tuberculosis. People with latent infection have a 5 to 10% lifetime risk of becoming ill with TB, representing a reservoir for TB active infection. This is a worrisome problem to overcome in the case of relapse; unfortunately, few drugs are effective against nonreplicating M. tuberculosis cells. Novel strategies to combat TB, including its latent form, are urgently needed. In response to the lack of new effective drugs and after screening about 500 original chemical molecules, we selected a compound, 11726172, that is endowed with potent antitubercular activity against M. tuberculosis both in vitro and in vivo and importantly also against dormant nonculturable bacilli. We also investigated the mechanism of action of 11726172 by applying a multidisciplinary approach, including transcriptomic, labeled metabolomic, biochemical, and microbiological procedures. Our results represent an important step forward in the development of a new antitubercular compound with a novel mechanism of action active against latent bacilli. IMPORTANCE The discontinuation of TB services due to COVID-19 causes concern about a future resurgence of TB, also considering that latent infection affects a high number of people worldwide. To combat this situation, the identification of antitubercular compounds targeting Mycobacterium tuberculosis through novel mechanisms of action is necessary. These compounds should be active against not only replicating bacteria cells but also nonreplicating cells to limit the reservoir of latently infected people on which the bacterium can rely to spread after reactivation.
Keywords: Mycobacterium tuberculosis; antitubercular drug; copper; latency; nonreplicating cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization. 2020. Global tuberculosis report 2020. World Health Organization, Geneva, Switzerland.
-
- Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, Blanc FX, Buonsenso D, Cho JG, Codecasa LR, Danila E, Duarte R, García-García JM, Gualano G, Rendon A, Silva DR, Souleymane MB, Tham SM, Thomas TA, Tiberi S, Udwadia ZF, Goletti D, Centis R, D'Ambrosio L, Sotgiu G, Ong CWM, Global Tuberculosis Network . 2021. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J 58:2101786. doi:10.1183/13993003.01786-2021. - DOI - PMC - PubMed
-
- Tiberi S, Vjecha MJ, Zumla A, Galvin J, Migliori GB, Zumla A. 2021. Accelerating development of new shorter TB treatment regimens in anticipation of a resurgence of multi-drug resistant TB due to the COVID-19 pandemic. Int J Infect Dis 113(Suppl 1):S96–S99. doi:10.1016/j.ijid.2021.02.067. - DOI - PMC - PubMed
-
- Roelens M, Battista Migliori G, Rozanova L, Estill J, Campbell JR, Cegielski JP, Tiberi S, Palmero D, Fox GJ, Guglielmetti L, Sotgiu G, Brust JCM, Bang D, Lienhardt C, Lange C, Menzies D, Keiser O, Raviglione M. 2021. Evidence-based definition for extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 204:713–722. doi:10.1164/rccm.202009-3527OC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
