Postinfluenza Environment Reduces Aspergillus fumigatus Conidium Clearance and Facilitates Invasive Aspergillosis In Vivo
- PMID: 36377895
- PMCID: PMC9765436
- DOI: 10.1128/mbio.02854-22
Postinfluenza Environment Reduces Aspergillus fumigatus Conidium Clearance and Facilitates Invasive Aspergillosis In Vivo
Abstract
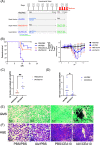
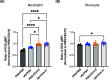
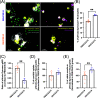
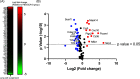
Aspergillus fumigatus is a human fungal pathogen that is most often avirulent in immunecompetent individuals because the innate immune system is efficient at eliminating fungal conidia. However, recent clinical observations have shown that severe influenza A virus (IAV) infection can lead to secondary A. fumigatus infections with high mortality. Little is currently known about how IAV infection alters the innate antifungal immune response. Here, we established a murine model of IAV-induced A. fumigatus (IAV-Af) superinfection by inoculating mice with IAV followed 6 days later by A. fumigatus conidia challenge. We observed increased mortality in the IAV-Af-superinfected mice compared to mice challenged with either IAV or A. fumigatus alone. A. fumigatus conidia were able to germinate and establish a biofilm in the lungs of the IAV-Af superinfection group, which was not seen following fungal challenge alone. While we did not observe any differences in inflammatory cell recruitment in the IAV-Af superinfection group compared to single-infection controls, we observed defects in Aspergillus conidial uptake and killing by both neutrophils and monocytes after IAV infection. pHrodo Green zymosan bioparticle (pHrodo-zymosan) and CM-H2DCFDA [5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate] staining, indicators of phagolysosome maturation and reactive oxygen species (ROS) production, respectively, revealed that the fungal killing defect was due in part to reduced phagolysosome maturation. Collectively, our data demonstrate that the ability of neutrophils and monocytes to kill and clear Aspergillus conidia is strongly reduced in the pulmonary environment of an IAV-infected lung, which leads to invasive pulmonary aspergillosis and increased overall mortality in our mouse model, recapitulating what is observed clinically in humans. IMPORTANCE Influenza A virus (IAV) is a common respiratory virus that causes seasonal illness in humans, but can cause pandemics and severe infection in certain patients. Since the emergence of the 2009 H1N1 pandemic strains, there has been an increase in clinical reports of IAV-infected patients in the intensive care unit (ICU) developing secondary pulmonary aspergillosis. These cases of flu-Aspergillus superinfections are associated with worse clinical outcomes than secondary bacterial infections in the setting of IAV. To date, we have a limited understanding of the cause(s) of secondary fungal infections in immunocompetent hosts. IAV-induced modulation of cytokine production and innate immune cellular function generates a unique immune environment in the lung, which could make the host vulnerable to a secondary fungal infection. Our work shows that defects in phagolysosome maturation in neutrophils and monocytes after IAV infection impair the ability of these cells to kill A. fumigatus, thus leading to increased fungal germination and growth and subsequent invasive aspergillosis. Our work lays a foundation for future mechanistic studies examining the exact immune modulatory events occurring in the respiratory tract after viral infection leading to secondary fungal infections.
Keywords: Aspergillus fumigatus; antifungal immunity; host-pathogen interactions; influenza A virus; innate immunity; invasive pulmonary aspergillosis; monocytes; neutrophils; pathogenesis; phagocytosis; phagolysosome; phagolysosome maturation; superinfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

