Fusarium graminearum Ste3 G-Protein Coupled Receptor: A Mediator of Hyphal Chemotropism and Pathogenesis
- PMID: 36377914
- PMCID: PMC9769807
- DOI: 10.1128/msphere.00456-22
Fusarium graminearum Ste3 G-Protein Coupled Receptor: A Mediator of Hyphal Chemotropism and Pathogenesis
Abstract
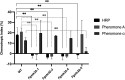
Fungal hyphal chemotropism has been shown to be a major contributor to host-pathogen interactions. Previous studies on Fusarium species have highlighted the involvement of the Ste2 G-protein-coupled receptor (GPCR) in mediating polarized hyphal growth toward host-released peroxidase. Here, the role of the opposite mating type GPCR, Ste3, is characterized with respect to Fusarium graminearum chemotropism and pathogenicity. Fgste3Δ deletion strains were found to be compromised in the chemotropic response toward peroxidase, development of lesions on germinating wheat, and infection of Arabidopsis thaliana leaves. In the absence of FgSte3 or FgSte2, F. graminearum cells exposed to peroxidase showed no phosphorylation of the cell-wall integrity, mitogen-activated protein kinase pathway component Mgv1. In addition, transcriptomic gene expression profiling yielded a list of genes involved in cellular reorganization, cell wall remodeling, and infection-mediated responses that were differentially modulated by peroxidase when FgSte3 was present. Deletion of FgSte3 yielded the downregulation of genes associated with mycotoxin biosynthesis and appressorium development, compared to the wild-type strain, both in the presence of peroxidase. Together, these findings contribute to our understanding of the mechanism underlying fungal chemotropism and pathogenesis while raising the novel hypothesis that FgSte2 and FgSte3 are interdependent on each other for the mediation of the redirection of hyphal growth in response to host-derived peroxidase. IMPORTANCE Fusarium head blight of wheat, caused by the filamentous fungus Fusarium graminearum, leads to devastating global food shortages and economic losses. Fungal hyphal chemotropism has been shown to be a major contributor to host-pathogen interactions. Here, the role of the opposite mating type GPCR, Ste3, is characterized with respect to F. graminearum chemotropism and pathogenicity. These findings contribute to our understanding of the mechanisms underlying fungal chemotropism and pathogenesis.
Keywords: Fusarium graminearum; Fusarium head blight; G-protein coupled receptors; hyphal chemotropism; pheromone receptor; wheat disease; wheat infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
