The Gut Microbiome Modulates Body Temperature Both in Sepsis and Health
- PMID: 36378114
- PMCID: PMC10112447
- DOI: 10.1164/rccm.202201-0161OC
The Gut Microbiome Modulates Body Temperature Both in Sepsis and Health
Abstract
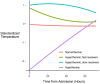
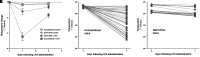
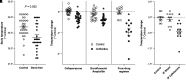
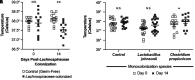
Rationale: Among patients with sepsis, variation in temperature trajectories predicts clinical outcomes. In healthy individuals, normal body temperature is variable and has decreased consistently since the 1860s. The biologic underpinnings of this temperature variation in disease and health are unknown. Objectives: To establish and interrogate the role of the gut microbiome in calibrating body temperature. Methods: We performed a series of translational analyses and experiments to determine whether and how variation in gut microbiota explains variation in body temperature in sepsis and in health. We studied patient temperature trajectories using electronic medical record data. We characterized gut microbiota in hospitalized patients using 16S ribosomal RNA gene sequencing. We modeled sepsis using intraperitoneal LPS in mice and modulated the microbiome using antibiotics, germ-free, and gnotobiotic animals. Measurements and Main Results: Consistent with prior work, we identified four temperature trajectories in patients hospitalized with sepsis that predicted clinical outcomes. In a separate cohort of 116 hospitalized patients, we found that the composition of patients' gut microbiota at admission predicted their temperature trajectories. Compared with conventional mice, germ-free mice had reduced temperature loss during experimental sepsis. Among conventional mice, heterogeneity of temperature response in sepsis was strongly explained by variation in gut microbiota. Healthy germ-free and antibiotic-treated mice both had lower basal body temperatures compared with control animals. The Lachnospiraceae family was consistently associated with temperature trajectories in hospitalized patients, experimental sepsis, and antibiotic-treated mice. Conclusions: The gut microbiome is a key modulator of body temperature variation in both health and critical illness and is thus a major, understudied target for modulating physiologic heterogeneity in sepsis.
Keywords: heterogeneity; microbiome; sepsis; subphenotypes; temperature.
Figures


Comment in
-
Good Bug, Bad Bug: What Is Influencing the Fever Response during Infection?Am J Respir Crit Care Med. 2023 Apr 15;207(8):967-969. doi: 10.1164/rccm.202211-2170ED. Am J Respir Crit Care Med. 2023. PMID: 36470241 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- K01 HL136687/HL/NHLBI NIH HHS/United States
- U01 AI124255/AI/NIAID NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- F31 HL158033/HL/NHLBI NIH HHS/United States
- R35 GM136312/GM/NIGMS NIH HHS/United States
- T32 HL007749/HL/NHLBI NIH HHS/United States
- T32 AI007528/AI/NIAID NIH HHS/United States
- R01 HL144599/HL/NHLBI NIH HHS/United States
- U19 AI090871/AI/NIAID NIH HHS/United States
- R01 AI143852/AI/NIAID NIH HHS/United States
- R01 HL158626/HL/NHLBI NIH HHS/United States
- R01 LM013325/LM/NLM NIH HHS/United States
- K24 HL159247/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

