TRAUMA INDUCES INTRAVASCULAR HEMOLYSIS, EXACERBATED BY RED BLOOD CELL TRANSFUSION AND ASSOCIATED WITH DISRUPTED ARGININE-NITRIC OXIDE METABOLISM
- PMID: 36378232
- PMCID: PMC9892361
- DOI: 10.1097/SHK.0000000000002036
TRAUMA INDUCES INTRAVASCULAR HEMOLYSIS, EXACERBATED BY RED BLOOD CELL TRANSFUSION AND ASSOCIATED WITH DISRUPTED ARGININE-NITRIC OXIDE METABOLISM
Abstract
Background: Severe injury can provoke systemic processes that lead to organ dysfunction, and hemolysis of both native and transfused red blood cells (RBCs) may contribute. Hemolysis can release erythrocyte proteins, such as hemoglobin and arginase-1, the latter with the potential to disrupt arginine metabolism and limit physiologic NO production. We aimed to quantify hemolysis and arginine metabolism in trauma patients and measure association with injury severity, transfusions, and outcomes. Methods: Blood was collected from injured patients at a level I trauma center enrolled in the COMBAT (Control of Major Bleeding After Trauma) trial. Proteomics and metabolomics were performed on plasma fractions through liquid chromatography coupled with mass spectrometry. Abundances of erythrocyte proteins comprising a hemolytic profile as well as haptoglobin, l -arginine, ornithine, and l -citrulline (NO surrogate marker) were analyzed at different timepoints and correlated with transfusions and adverse outcomes. Results: More critically injured patients, nonsurvivors, and those with longer ventilator requirement had higher levels of hemolysis markers with reduced l -arginine and l -citrulline. In logistic regression, elevated hemolysis markers, reduced l -arginine, and reduced l -citrulline were significantly associated with these adverse outcomes. An increased number of blood transfusions were significantly associated with elevated hemolysis markers and reduced l -arginine and l -citrulline independently of New Injury Severity Score and arterial base excess. Conclusions: Severe injury induces intravascular hemolysis, which may mediate postinjury organ dysfunction. In addition to native RBCs, transfused RBCs can lyse and may exacerbate trauma-induced hemolysis. Arginase-1 released from RBCs may contribute to the depletion of l -arginine and the subsequent reduction in the NO necessary to maintain organ perfusion.
Copyright © 2022 by the Shock Society.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
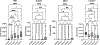



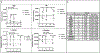
References
-
- Hardaway RM, Johnson DG, Elovitz MJ, Houchin DN, Jenkins EB, Burns JW, Jackson DR. Influence of trauma and hemolysis on hemorrhagic shock in dogs. J Trauma. 4:624–641, 1964. - PubMed
-
- Stahl WM. Acute phase protein response to tissue injury. Crit Care Med. 15(6):545–550, 1987. - PubMed
-
- Gando S, Tedo I. The effects of massive transfusion and haptoglobin therapy on hemolysis in trauma patients. Surg Today. 24(9):785–790, 1994. - PubMed
-
- Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, Sauaia A, West B, Banerjee A, Silliman CC. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 43(1):39–46, 2015. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

