A BioBricks Metabolic Engineering Platform for the Biosynthesis of Anthracyclinones in Streptomyces coelicolor
- PMID: 36378506
- PMCID: PMC9764417
- DOI: 10.1021/acssynbio.2c00498
A BioBricks Metabolic Engineering Platform for the Biosynthesis of Anthracyclinones in Streptomyces coelicolor
Abstract
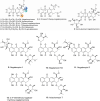
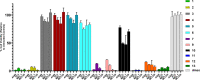
Actinomycetes produce a variety of clinically indispensable molecules, such as antineoplastic anthracyclines. However, the actinomycetes are hindered in their further development as genetically engineered hosts for the synthesis of new anthracycline analogues due to their slow growth kinetics associated with their mycelial life cycle and the lack of a comprehensive genetic toolbox for combinatorial biosynthesis. In this report, we tackled both issues via the development of the BIOPOLYMER (BIOBricks POLYketide Metabolic EngineeRing) toolbox: a comprehensive synthetic biology toolbox consisting of engineered strains, promoters, vectors, and biosynthetic genes for the synthesis of anthracyclinones. An improved derivative of the production host Streptomyces coelicolor M1152 was created by deleting the matAB gene cluster that specifies extracellular poly-β-1,6-N-acetylglucosamine (PNAG). This resulted in a loss of mycelial aggregation, with improved biomass accumulation and anthracyclinone production. We then leveraged BIOPOLYMER to engineer four distinct anthracyclinone pathways, identifying optimal combinations of promoters, genes, and vectors to produce aklavinone, 9-epi-aklavinone, auramycinone, and nogalamycinone at titers between 15-20 mg/L. Optimization of nogalamycinone production strains resulted in titers of 103 mg/L. We structurally characterized six anthracyclinone products from fermentations, including new compounds 9,10-seco-7-deoxy-nogalamycinone and 4-O-β-d-glucosyl-nogalamycinone. Lastly, we tested the antiproliferative activity of the anthracyclinones in a mammalian cancer cell viability assay, in which nogalamycinone, auramycinone, and aklavinone exhibited moderate cytotoxicity against several cancer cell lines. We envision that BIOPOLYMER will serve as a foundational platform technology for the synthesis of designer anthracycline analogues.
Keywords: BioBricks; Streptomyces coelicolor; anthracyclinones; anticancer; natural product biosynthesis; synthetic biology.
Conflict of interest statement
The authors declare the following competing financial interest(s): Material published in this report is covered under U.S. Patent Application No. 16/015,821 to Ferris State University.
Figures







Similar articles
-
Diverse Combinatorial Biosynthesis Strategies for C-H Functionalization of Anthracyclinones.ACS Synth Biol. 2024 May 17;13(5):1523-1536. doi: 10.1021/acssynbio.4c00043. Epub 2024 Apr 25. ACS Synth Biol. 2024. PMID: 38662967 Free PMC article.
-
A BioBricks toolbox for metabolic engineering of the tetracenomycin pathway.Biotechnol J. 2022 Mar;17(3):e2100371. doi: 10.1002/biot.202100371. Epub 2021 Nov 12. Biotechnol J. 2022. PMID: 34719127 Free PMC article.
-
Discovery, characterization, and engineering of an advantageous Streptomyces host for heterologous expression of natural product biosynthetic gene clusters.Microb Cell Fact. 2024 May 24;23(1):149. doi: 10.1186/s12934-024-02416-y. Microb Cell Fact. 2024. PMID: 38790014 Free PMC article.
-
[Efficient production of polyketide products in Streptomyces hosts - A review].Wei Sheng Wu Xue Bao. 2016 Mar 4;56(3):418-28. Wei Sheng Wu Xue Bao. 2016. PMID: 27382785 Review. Chinese.
-
Synthetic biology enabling access to designer polyketides.Curr Opin Chem Biol. 2020 Oct;58:45-53. doi: 10.1016/j.cbpa.2020.06.003. Epub 2020 Aug 4. Curr Opin Chem Biol. 2020. PMID: 32758909 Free PMC article. Review.
Cited by
-
Biochemical and Structural Studies of the Carminomycin 4-O-Methyltransferase DnrK.J Nat Prod. 2024 Apr 26;87(4):798-809. doi: 10.1021/acs.jnatprod.3c00947. Epub 2024 Feb 27. J Nat Prod. 2024. PMID: 38412432 Free PMC article.
-
Engineered CRISPR-Cas9 for Streptomyces sp. genome editing to improve specialized metabolite production.Nat Commun. 2025 Jan 21;16(1):874. doi: 10.1038/s41467-025-56278-y. Nat Commun. 2025. PMID: 39833194 Free PMC article.
-
Engineering the next generation of theranostic biomaterials with synthetic biology.Bioact Mater. 2023 Nov 3;32:514-529. doi: 10.1016/j.bioactmat.2023.10.018. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 38026437 Free PMC article. Review.
-
Rethinking Biosynthesis of Aclacinomycin A.Molecules. 2023 Mar 18;28(6):2761. doi: 10.3390/molecules28062761. Molecules. 2023. PMID: 36985733 Free PMC article. Review.
-
Current Approaches for Genetic Manipulation of Streptomyces spp.-Key Bacteria for Biotechnology and Environment.BioTech (Basel). 2025 Jan 2;14(1):3. doi: 10.3390/biotech14010003. BioTech (Basel). 2025. PMID: 39846552 Free PMC article. Review.
References
-
- Pang B.; Qiao X.; Janssen L.; Velds A.; Groothuis T.; Kerkhoven R.; Nieuwland M.; Ovaa H.; Rottenberg S.; van Tellingen O.; Janssen J.; Huijgens P.; Zwart W.; Neefjes J. Drug-Induced Histone Eviction from Open Chromatin Contributes to the Chemotherapeutic Effects of Doxorubicin. Nat. Commun. 2013, 4 (1), 1–13. 10.1038/ncomms2921. - DOI - PMC - PubMed
-
- Torkkell S.; Kunnari T.; Palmu K.; Mäntsälä P.; Hakala J.; Ylihonko K. The Entire Nogalamycin Biosynthetic Gene Cluster of Streptomyces nogalater: Characterization of a 20-Kb DNA Region and Generation of Hybrid Structures. Molecular Genetics and Genomics 2001, 266 (2), 276–288. 10.1007/s004380100554. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

