Intra-annual fluctuation in morphology and microfibril angle of tracheids revealed by novel microscopy-based imaging
- PMID: 36378676
- PMCID: PMC9665381
- DOI: 10.1371/journal.pone.0277616
Intra-annual fluctuation in morphology and microfibril angle of tracheids revealed by novel microscopy-based imaging
Abstract
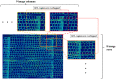
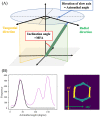
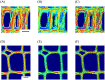
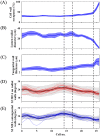
Woody cells, such as tracheids, fibers, vessels, rays etc., have unique structural characteristics such as nano-scale ultrastructure represented by multilayers, microfibril angle (MFA), micro-scale anatomical properties and spatial arrangement. Simultaneous evaluation of the above indices is very important for their adequate quantification and extracting the effects of external stimuli from them. However, it is difficult in general to achieve the above only by traditional methodologies. To overcome the above point, a new methodological framework combining polarization optical microscopy, fluorescence microscopy, and image segmentation is proposed. The framework was tested to a model softwood species, Chamaecyparis obtusa for characterizing intra-annual transition of MFA and tracheid morphology in a radial file unit. According our result, this framework successfully traced the both characteristics tracheid by tracheid and revealed the high correlation (|r| > 0.5) between S2 microfibril angles and tracheidal morphology (lumen radial diameter, tangential wall thickness and cell wall occupancy). In addition, radial file based evaluation firstly revealed their complex transitional behavior in transition and latewood. The proposed framework has great potential as one of the unique tools to provide detailed insights into heterogeneity of intra and inter-cells in the wide field of view through the simultaneous evaluation of cells' ultrastructure and morphological properties.
Copyright: © 2022 Kita et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Côté WA. Cellular ultrastructure of woody plants. New York: Syracuse University Press; 1965.
-
- Donaldson L. Wood cell wall ultrastructure The key to understanding wood properties and behavior. IAWA J. 2019; 40: 645–672.
-
- Donaldson L, Xu P. Microfibril orientation across the secondary cell wall of Radiata pine tracheids. Trees 2004; 19-644-653.
-
- Donaldson L. Microfibril angle: measurement, variation and relationships—a review. IAWA J. 2008; 29: 345–386.
-
- Richter HG, Grosser D, Heinz I, Gasson PE. IAWA list of microscopic features for softwood identification. IAWA J. 2004; 25: 1–70.

