On-demand cell-autonomous gene therapy for brain circuit disorders
- PMID: 36378958
- PMCID: PMC7613996
- DOI: 10.1126/science.abq6656
On-demand cell-autonomous gene therapy for brain circuit disorders
Abstract
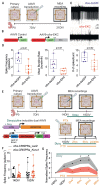
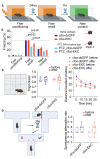
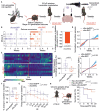
Several neurodevelopmental and neuropsychiatric disorders are characterized by intermittent episodes of pathological activity. Although genetic therapies offer the ability to modulate neuronal excitability, a limiting factor is that they do not discriminate between neurons involved in circuit pathologies and "healthy" surrounding or intermingled neurons. We describe a gene therapy strategy that down-regulates the excitability of overactive neurons in closed loop, which we tested in models of epilepsy. We used an immediate early gene promoter to drive the expression of Kv1.1 potassium channels specifically in hyperactive neurons, and only for as long as they exhibit abnormal activity. Neuronal excitability was reduced by seizure-related activity, leading to a persistent antiepileptic effect without interfering with normal behaviors. Activity-dependent gene therapy is a promising on-demand cell-autonomous treatment for brain circuit disorders.
Conflict of interest statement
Figures



Comment in
-
Gene therapy for epilepsy.Science. 2022 Nov 4;378(6619):471-472. doi: 10.1126/science.ade8836. Epub 2022 Nov 3. Science. 2022. PMID: 36378973
-
'On-demand' gene therapy for epilepsy.Nat Rev Neurol. 2023 Mar;19(3):130-131. doi: 10.1038/s41582-022-00761-3. Nat Rev Neurol. 2023. PMID: 36509840 No abstract available.
-
Only Hit the Bad Guys: A Gene Therapy Approach to Selectively Silence Highly Active Neurons Reduces Chronic Seizures in Epileptic Mice.Epilepsy Curr. 2023 Dec 12;24(1):56-58. doi: 10.1177/15357597231210253. eCollection 2024 Jan-Feb. Epilepsy Curr. 2023. PMID: 38327536 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

