Trastuzumab Deruxtecan in Anti-Human Epidermal Growth Factor Receptor 2 Treatment-Naive Patients With Human Epidermal Growth Factor Receptor 2-Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial
- PMID: 36379002
- PMCID: PMC9901967
- DOI: 10.1200/JCO.22.00575
Trastuzumab Deruxtecan in Anti-Human Epidermal Growth Factor Receptor 2 Treatment-Naive Patients With Human Epidermal Growth Factor Receptor 2-Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial
Abstract
Purpose: To investigate efficacy and safety of trastuzumab deruxtecan (T-DXd) in human epidermal growth factor receptor 2 (HER2)-low gastric or gastroesophageal junction (GEJ) adenocarcinoma.
Methods: Patients with locally advanced or metastatic HER2-low (cohort 1, immunohistochemistry 2+/in situ hybridization-negative; cohort 2, immunohistochemistry 1+) gastric/GEJ adenocarcinoma treated with at least two prior regimens, including fluoropyrimidine and platinum, but anti-HER2 therapy naive, received T-DXd 6.4 mg/kg intravenously once every 3 weeks. The primary end point was confirmed objective response rate by independent central review.
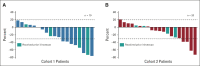
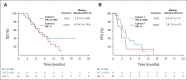
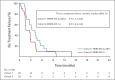
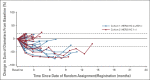
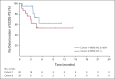
Results: Among 21 patients enrolled in cohort 1 and 24 enrolled in cohort 2, 19 and 21 patients, respectively, had central HER2 confirmation, received T-DXd, and had measurable tumors at baseline. The confirmed objective response rate was 26.3% (95% CI, 9.1 to 51.2) from five partial responses in cohort 1 and 9.5% (95% CI, 1.2 to 30.4) from two partial responses in cohort 2. Thirteen patients (68.4%) in cohort 1 and 12 (60.0%) in cohort 2 experienced reduced tumor size. The median overall survival was 7.8 months (95% CI, 4.7 to nonevaluable) in cohort 1 and 8.5 months (95% CI, 4.3 to 10.9) in cohort 2; the median progression-free survival was 4.4 months (95% CI, 2.7 to 7.1) and 2.8 months (95% CI, 1.5 to 4.3), respectively. The most common grade ≥ 3 treatment-emergent adverse events in cohorts 1 and 2 were anemia (30.0% and 29.2%), decreased neutrophil count (25.0% and 29.2%), and decreased appetite (20.0% and 20.8%). Drug-related interstitial lung disease/pneumonitis occurred in one patient in each cohort (grade 1 or 2). No drug-related deaths occurred.
Conclusion: This study provides preliminary evidence that T-DXd has clinical activity in patients with heavily pretreated HER2-low gastric/GEJ adenocarcinoma.
Trial registration: ClinicalTrials.gov NCT03329690.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
References
-
- Aoki M, Iwasa S, Boku N: Trastuzumab deruxtecan for the treatment of HER2-positive advanced gastric cancer: A clinical perspective. Gastric Cancer 24:567-576, 2021 - PubMed
-
- Pietrantonio F, Caporale M, Morano F, et al. : HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer 139:2859-2864, 2016 - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

