Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina
- PMID: 36379210
- PMCID: PMC9772037
- DOI: 10.1016/j.immuni.2022.10.018
Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina
Abstract
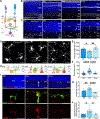
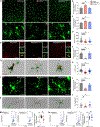
Microglia utilize their phagocytic activity to prune redundant synapses and refine neural circuits during precise developmental periods. However, the neuronal signals that control this phagocytic clockwork remain largely undefined. Here, we show that neuronal signal-regulatory protein alpha (SIRPα) is a permissive cue for microglial phagocytosis in the developing murine retina. Removal of neuronal, but not microglial, SIRPα reduced microglial phagocytosis, increased synpase numbers, and impaired circuit function. Conversely, prolonging neuronal SIRPα expression extended developmental microglial phagocytosis. These outcomes depended on the interaction of presynaptic SIRPα with postsynaptic CD47. Global CD47 deficiency modestly increased microglial phagocytosis, while CD47 overexpression reduced it. This effect was rescued by coexpression of neuronal SIRPα or codeletion of neuronal SIRPα and CD47. These data indicate that neuronal SIRPα regulates microglial phagocytosis by limiting microglial SIRPα access to neuronal CD47. This discovery may aid our understanding of synapse loss in neurological diseases.
Keywords: SIRPα; microglia; retina; synapse refinement.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Dopp EA, Dijkstra CD, Simmons DL, and van den Berg TK (1998). Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol 161, 1853–1859. - PubMed
-
- Azcutia V, Bassil R, Herter JM, Engelbertsen D, Newton G, Autio A, Mayadas T, Lichtman AH, Khoury SJ, Parkos CA, et al. (2017). Defects in CD4+ T cell LFA-1 integrin-dependent adhesion and proliferation protect Cd47−/− mice from EAE. J Leukoc Biol 101, 493–505. 10.1189/jlb.3A1215-546RR. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY030458/EY/NEI NIH HHS/United States
- DP2 EY027984/EY/NEI NIH HHS/United States
- R21 AG074163/AG/NIA NIH HHS/United States
- R56 AG061808/AG/NIA NIH HHS/United States
- T32 EY007001/EY/NEI NIH HHS/United States
- R01 EY035254/EY/NEI NIH HHS/United States
- U54 HG006348/HG/NHGRI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- R01 NS117533/NS/NINDS NIH HHS/United States
- T32 GM088129/GM/NIGMS NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01 MH113743/MH/NIMH NIH HHS/United States
- UM1 HG006348/HG/NHGRI NIH HHS/United States
- U42 OD011174/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

