Clinical and immune response characteristics among vaccinated persons infected with SARS-CoV-2 delta variant: a retrospective study
- PMID: 36379610
- PMCID: PMC9676093
- DOI: 10.1631/jzus.B2200054
Clinical and immune response characteristics among vaccinated persons infected with SARS-CoV-2 delta variant: a retrospective study
Abstract
Objectives: This study aimed to observe the clinical and immune response characteristics of vaccinated persons infected with the delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Yangzhou, China.
Methods: We extracted the medical data of 129 patients with delta-variant infection who were admitted to Northern Jiangsu People's Hospital (Yangzhou, China) between August and September, 2021. The patients were grouped according to the number of vaccine doses received into an unvaccinated group: a one-dose group and a two-dose group. The vaccine used was SARS-CoV-2-inactivated vaccine developed by Sinovac. We retrospectively analyzed the patients' epidemiological, clinical, laboratory, and imaging data.
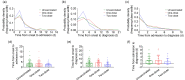
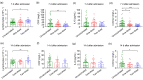
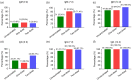
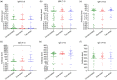
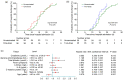
Results: Almost all patients with delta-variant infection in Yangzhou were elderly, and patients with severe/critical illness were over 70 years of age. The rates of severe/critical illness (P=0.006), fever (P=0.025), and dyspnea (P=0.045) were lower in the two-dose group than in the unvaccinated group. Compared to the unvaccinated group, the two-dose group showed significantly higher lymphocyte counts and significantly lower levels of C-reactive protein (CRP), interleukin-6 (IL-6), and D-dimer during hospitalization and a significantly higher positive rate of immunoglobulin G (IgG) antibodies at admission (all P<0.05). The cumulative probabilities of hospital discharge and negative virus conversion were also higher in the two-dose group than in the unvaccinated group (P<0.05).
Conclusions: Two doses of the SARS-CoV-2-inactivated vaccine were highly effective at limiting symptomatic disease and reducing immune response, while a single dose did not seem to be effective.
Keywords: Delta variant; Hospitalization; Immune response; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Vaccine.
Figures
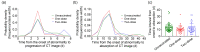

Similar articles
-
[Clinical features of children with coronavirus disease 2019 Delta variant infection after vaccination with inactivated SARS-CoV-2 vaccine].Zhongguo Dang Dai Er Ke Za Zhi. 2022 Jul 15;24(7):742-747. doi: 10.7499/j.issn.1008-8830.2203174. Zhongguo Dang Dai Er Ke Za Zhi. 2022. PMID: 35894187 Free PMC article. Chinese.
-
[Effects of vaccination status on the disease severity of patients with coronavirus disease 2019].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Sep;34(9):915-920. doi: 10.3760/cma.j.cn121430-20220318-00264. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36377443 Chinese.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Disease severity and efficacy of homologous vaccination among patients infected with SARS-CoV-2 Delta or Omicron VOCs, compared to unvaccinated using main biomarkers.J Med Virol. 2022 Dec;94(12):5867-5876. doi: 10.1002/jmv.28098. Epub 2022 Sep 9. J Med Virol. 2022. PMID: 36029103 Free PMC article.
-
A systematic review of Vaccine Breakthrough Infections by SARS-CoV-2 Delta Variant.Int J Biol Sci. 2022 Jan 1;18(2):889-900. doi: 10.7150/ijbs.68973. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002532 Free PMC article.
Cited by
-
Assessing the Association between Biomarkers and COVID-19 Mortality Using the Joint Modelling Approach.Life (Basel). 2024 Mar 6;14(3):343. doi: 10.3390/life14030343. Life (Basel). 2024. PMID: 38541668 Free PMC article.
-
Evolution of the newest diagnostic methods for COVID-19: a Chinese perspective.J Zhejiang Univ Sci B. 2023 Jun 15;24(6):463-484. doi: 10.1631/jzus.B2200625. J Zhejiang Univ Sci B. 2023. PMID: 37309039 Free PMC article. Review.
-
Effect of COVID-19 vaccine in adults infected with the Delta variant of SARS-CoV-2: a retrospective cohort study.J Thorac Dis. 2024 Oct 31;16(10):6983-6998. doi: 10.21037/jtd-24-1351. Epub 2024 Oct 15. J Thorac Dis. 2024. PMID: 39552877 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 82171207 and 82172190/the National Natural Science Foundation of China
- 2021-008/the Jiangsu Association for Science and Technology Young Scientific and Technological Talents Support Project
- 2022-3-6-146/the Jiangsu Province "333" High-level Talents Training Project
- YZ2021088 and YZ2021148/the Yangzhou Science and Technology Plan Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

