Fast Synaptically Activated Calcium and Sodium Kinetics in Hippocampal Pyramidal Neuron Dendritic Spines
- PMID: 36379712
- PMCID: PMC9718353
- DOI: 10.1523/ENEURO.0396-22.2022
Fast Synaptically Activated Calcium and Sodium Kinetics in Hippocampal Pyramidal Neuron Dendritic Spines
Abstract
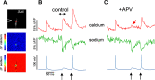
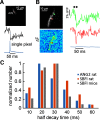
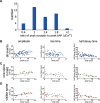
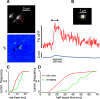
An accurate assessment of the time course, components, and magnitude of postsynaptic currents is important for a quantitative understanding of synaptic integration and signaling in dendritic spines. These parameters have been studied in some detail in previous experiments, primarily using two-photon imaging of [Ca2+]i changes and two-photon uncaging of glutamate. However, even with these revolutionary techniques, there are some missing pieces in our current understanding, particularly related to the time courses of synaptically evoked [Ca2+]i and [Na+]i changes. In new experiments, we used low-affinity, linear Na+ and Ca2+ indicators, laser fluorescence stimulation, and a sensitive camera-based detection system, combined with electrical stimulation and two-photon glutamate uncaging, to extend measurements of these spine parameters. We found that (1) almost all synaptically activated Na+ currents in CA1 hippocampal pyramidal neuron spines in slices from mice of either sex are through AMPA receptors with little Na+ entry through voltage-gated sodium channels (VGSCs) or NMDA receptor channels; (2) a spectrum of sodium transient decay times was observed, suggesting a spectrum of spine neck resistances, even on the same dendrite; (3) synaptically activated [Ca2+]i changes are very fast and are almost entirely because of Ca2+ entry through NMDA receptors at the time when the Mg2+ block is relieved by the fast AMPA-mediated EPSP; (4) the [Ca2+]i changes evoked by uncaging glutamate are slower than the changes evoked by synaptic release, suggesting that the relative contribution of Ca2+ entering through NMDA receptors at rest following uncaging is higher than following electrical stimulation.
Keywords: AMPA; NMDA; calcium; sodium; spine.
Copyright © 2022 Miyazaki and Ross.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Electrical properties of dendritic spines.Biophys J. 2023 Nov 21;122(22):4303-4315. doi: 10.1016/j.bpj.2023.10.008. Epub 2023 Oct 13. Biophys J. 2023. PMID: 37837192 Free PMC article. Review.
-
Sodium Dynamics in Pyramidal Neuron Dendritic Spines: Synaptically Evoked Entry Predominantly through AMPA Receptors and Removal by Diffusion.J Neurosci. 2017 Oct 11;37(41):9964-9976. doi: 10.1523/JNEUROSCI.1758-17.2017. Epub 2017 Sep 13. J Neurosci. 2017. PMID: 28904093 Free PMC article.
-
Impact of subthreshold membrane potential on synaptic responses at dendritic spines of layer 5 pyramidal neurons in the prefrontal cortex.J Neurophysiol. 2014 May;111(10):1960-72. doi: 10.1152/jn.00590.2013. Epub 2014 Jan 29. J Neurophysiol. 2014. PMID: 24478153 Free PMC article.
-
Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines.Neuron. 2007 Jan 18;53(2):249-60. doi: 10.1016/j.neuron.2006.12.017. Neuron. 2007. PMID: 17224406
-
Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels.J Physiol. 2008 Mar 15;586(6):1475-80. doi: 10.1113/jphysiol.2007.148353. Epub 2007 Dec 20. J Physiol. 2008. PMID: 18096597 Free PMC article. Review.
Cited by
-
A generalized mathematical framework for the calcium control hypothesis describes weight-dependent synaptic plasticity.J Comput Neurosci. 2025 Jun;53(2):333-357. doi: 10.1007/s10827-025-00894-6. Epub 2025 Mar 18. J Comput Neurosci. 2025. PMID: 40100329 Free PMC article.
-
Electrophysiology of Dendritic Spines: Information Processing, Dynamic Compartmentalization, and Synaptic Plasticity.Adv Neurobiol. 2023;34:103-141. doi: 10.1007/978-3-031-36159-3_3. Adv Neurobiol. 2023. PMID: 37962795
-
Neuronal Imaging at 8-Bit Depth to Combine High Spatial and High Temporal Resolution With Acquisition Rates Up To 40 kHz.J Biophotonics. 2025 Mar;18(3):e202400513. doi: 10.1002/jbio.202400513. Epub 2025 Jan 10. J Biophotonics. 2025. PMID: 39791263 Free PMC article.
-
Electrical properties of dendritic spines.Biophys J. 2023 Nov 21;122(22):4303-4315. doi: 10.1016/j.bpj.2023.10.008. Epub 2023 Oct 13. Biophys J. 2023. PMID: 37837192 Free PMC article. Review.
-
Comparing confocal and two-photon Ca2+ imaging of thin low-scattering preparations.Biophys Rep (N Y). 2023 Apr 20;3(2):100109. doi: 10.1016/j.bpr.2023.100109. eCollection 2023 Jun 14. Biophys Rep (N Y). 2023. PMID: 37213258 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
