Burden of medically attended influenza infection and cases averted by vaccination - United States, 2016/17 through 2018/19 influenza seasons
- PMID: 36379754
- PMCID: PMC9732931
- DOI: 10.1016/j.vaccine.2022.11.011
Burden of medically attended influenza infection and cases averted by vaccination - United States, 2016/17 through 2018/19 influenza seasons
Abstract
Background: Epidemics of seasonal influenza vary in intensity annually, and influenza vaccine effectiveness (VE) fluctuates based in part on antigenic match to circulating viruses. We estimated the incidence of influenza and influenza cases averted by vaccination in four ambulatory care sites in the United States, during seasons when overall influenza VE ranged from 29% to 40%.
Methods: We conducted active surveillance for influenza at ambulatory care settings at four sites within the United States Influenza Vaccine Effectiveness Network. We extrapolated the total number of influenza cases in the source populations served by these organizations based on incidence of medically attended acute respiratory illness in the source population and influenza test results in those actively tested for influenza. We estimated the number of medically attended influenza cases averted based on incidence, vaccine coverage, and VE.
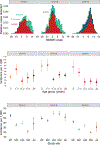
Results: From 2016/17 through 2018/19, incidence of ambulatory visits for laboratory-confirmed influenza ranged from 31 to 51 per 1,000 population. Incidence was highest in children aged 9-17 years (range, 56 to 81 per 1,000) and lowest in adults aged 18-49 years (range, 23-32 per 1,000). Medically attended cases averted by vaccination ranged from a high of 46.6 (95 % CI, 12.1- 91.9) per 1,000 vaccinees in children aged 6 months to 8 years, to a low of 6.9 (95 % CI, -5.1- 27.3) per 1,000 vaccinees in adults aged ≥ 65 years.
Discussion: Even in seasons with low vaccine effectiveness for a particular virus subtype, influenza vaccines can still lead to clinically meaningful reductions in ambulatory care visits for influenza.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

