17β-estradiol ameliorates delirium-like phenotypes in a murine model of urinary tract infection
- PMID: 36380004
- PMCID: PMC9666646
- DOI: 10.1038/s41598-022-24247-w
17β-estradiol ameliorates delirium-like phenotypes in a murine model of urinary tract infection
Abstract
Urinary tract infections (UTIs) are common and frequently precipitate delirium-like states. Advanced age coincident with the postmenopausal period is a risk factor for delirium following UTIs. We previously demonstrated a pathological role for interleukin-6 (IL-6) in mediating delirium-like phenotypes in a murine model of UTI. Estrogen has been implicated in reducing peripheral IL-6 expression, but it is unknown whether the increased susceptibility of postmenopausal females to developing delirium concomitant with UTIs reflects diminished effects of circulating estrogen. Here, we tested this hypothesis in a mouse model of UTI. Female C57BL/6J mice were oophorectomized, UTIs induced by transurethral inoculation of E. coli, and treated with 17β-estradiol. Delirium-like behaviors were evaluated prior to and following UTI and 17β-estradiol treatment. Compared to controls, mice treated with 17β-estradiol had less neuronal injury, improved delirium-like behaviors, and less plasma and frontal cortex IL-6. In vitro studies further showed that 17β-estradiol may also directly mediate neuronal protection, suggesting pleiotropic mechanisms of 17β-estradiol-mediated neuroprotection. In summary, we demonstrate a beneficial role for 17β-estradiol in ameliorating acute UTI-induced structural and functional delirium-like phenotypes. These findings provide pre-clinical justification for 17β-estradiol as a therapeutic target to ameliorate delirium following UTI.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
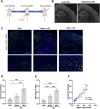

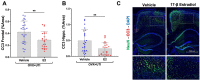
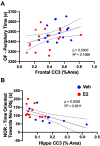



References
-
- Chae JH, Miller BJ. Beyond urinary tract infections (UTIs) and delirium: A systematic review of UTIs and neuropsychiatric disorders. J. Psychiatr. Pract. 2015;21(6):402–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

