Presepsin levels and COVID-19 severity: a systematic review and meta-analysis
- PMID: 36380007
- PMCID: PMC9666937
- DOI: 10.1007/s10238-022-00936-8
Presepsin levels and COVID-19 severity: a systematic review and meta-analysis
Abstract
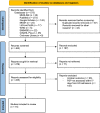
Plasmatic presepsin (PSP) is a novel biomarker reported to be useful for sepsis diagnosis and prognosis. During the pandemic, only few studies highlighted a possible correlation between PSP and COVID-19 severity, but results remain inconsistent. The present study aims to establish the correlation between PSP and COVID-19 severity. English-language papers assessing a correlation between COVID-19 and PSP from MEDLINE, PubMed, Google Scholar, Cochrane Library, MeSH, LitCovid NLM, EMBASE, CINAHL Plus and the World Health Organization (WHO) website, published from January 2020 were considered with no publication date limitations. Two independent reviewers performed data abstraction and quality assessment, and one reviewer resolved inconsistencies. The protocol was registered on PROSPERO (CRD42022325971).Fifteen articles met our eligibility criteria. The aggregate study population included 1373 COVID-19 patients who had undergone a PSP assessment. The random-effect meta-analysis was performed in 7 out of 15 selected studies, considering only those reporting the mean PSP levels in low- and high-severity cases (n = 707).The results showed that the pooled mean difference of PSP levels between high- and low-severity COVID-19 patients was 441.70 pg/ml (95%CI: 150.40-732.99 pg/ml).Our data show that presepsin is a promising biomarker that can express COVID-19 severity.
Keywords: COVID-19; Disease severity; Presepsin; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
Authors have no conflicts of interest to disclose.
Figures
References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- World Health Organisation. https://covid19.who.int/ (accessed 09 June 2022).
-
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. - DOI - PMC - PubMed
-
- Rotondo JC, Martini F, Maritati M, Mazziotta C, Di Mauro G, Lanzillotti C, Barp N, Gallerani A, Tognon M, Contini C. SARS-CoV2 infection: new molecula, phylogenetic and pathogenetic insight efficacy of current vaccines and the potential risk of variants. Viruses. 2021;13:1687. doi: 10.3390/v13091687. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous