Death by TNF: a road to inflammation
- PMID: 36380021
- PMCID: PMC9665039
- DOI: 10.1038/s41577-022-00792-3
Death by TNF: a road to inflammation
Abstract
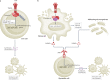
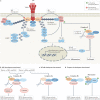
Tumour necrosis factor (TNF) is a central cytokine in inflammatory reactions, and biologics that neutralize TNF are among the most successful drugs for the treatment of chronic inflammatory and autoimmune pathologies. In recent years, it became clear that TNF drives inflammatory responses not only directly by inducing inflammatory gene expression but also indirectly by inducing cell death, instigating inflammatory immune reactions and disease development. Hence, inhibitors of cell death are being considered as a new therapy for TNF-dependent inflammatory diseases.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Shear MJ, Perrault A. Chemical treatment of tumors. IX. Reactions of mice with primary subcutaneous tumors to injection of a hemorrhage-producing bacterial polysaccharide. J. Natl Cancer Inst. 1944;44:461–476.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

