TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective
- PMID: 36380023
- PMCID: PMC10634249
- DOI: 10.1038/s41577-022-00796-z
TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective
Abstract
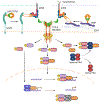
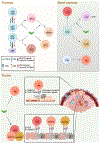
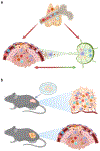
The immune system responds to cancer in two main ways. First, there are prewired responses involving myeloid cells, innate lymphocytes and innate-like adaptive lymphocytes that either reside in premalignant tissues or migrate directly to tumours, and second, there are antigen priming-dependent responses, in which adaptive lymphocytes are primed in secondary lymphoid organs before homing to tumours. Transforming growth factor-β (TGFβ) - one of the most potent and pleiotropic regulatory cytokines - controls almost every stage of the tumour-elicited immune response, from leukocyte development in primary lymphoid organs to their priming in secondary lymphoid organs and their effector functions in the tumour itself. The complexity of TGFβ-regulated immune cell circuitries, as well as the contextual roles of TGFβ signalling in cancer cells and tumour stromal cells, necessitates the use of rigorous experimental systems that closely recapitulate human cancer, such as autochthonous tumour models, to uncover the underlying immunobiology. The diverse functions of TGFβ in healthy tissues further complicate the search for effective and safe cancer therapeutics targeting the TGFβ pathway. Here we discuss the contextual complexity of TGFβ signalling in tumour-elicited immune responses and explain how understanding this may guide the development of mechanism-based cancer immunotherapy.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing Interests
Memorial Sloan Kettering Cancer Center owns a patent on “Methods and Compositions For Targeting TGF-β Signaling in CD4+ Helper T Cells for Cancer Immunotherapy” with M.L. listed as an inventor.
Figures

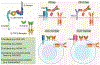
Similar articles
-
Overcoming TGFβ-mediated immune evasion in cancer.Nat Rev Cancer. 2022 Jan;22(1):25-44. doi: 10.1038/s41568-021-00413-6. Epub 2021 Oct 20. Nat Rev Cancer. 2022. PMID: 34671117 Review.
-
TGFβ biology in cancer progression and immunotherapy.Nat Rev Clin Oncol. 2021 Jan;18(1):9-34. doi: 10.1038/s41571-020-0403-1. Epub 2020 Jul 24. Nat Rev Clin Oncol. 2021. PMID: 32710082 Free PMC article. Review.
-
TGFbeta signalling: a complex web in cancer progression.Nat Rev Cancer. 2010 Jun;10(6):415-24. doi: 10.1038/nrc2853. Nat Rev Cancer. 2010. PMID: 20495575 Review.
-
Saga of monokines in shaping tumour-immune microenvironment: Origin to execution.Cytokine. 2022 Sep;157:155948. doi: 10.1016/j.cyto.2022.155948. Epub 2022 Jun 25. Cytokine. 2022. PMID: 35764025 Review.
-
TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis.Nature. 2018 Feb 22;554(7693):538-543. doi: 10.1038/nature25492. Epub 2018 Feb 14. Nature. 2018. PMID: 29443964
Cited by
-
Molecular determinants of immunogenic cell death elicited by radiation therapy.Immunol Rev. 2024 Jan;321(1):20-32. doi: 10.1111/imr.13271. Epub 2023 Sep 7. Immunol Rev. 2024. PMID: 37679959 Free PMC article. Review.
-
From barriers to novel strategies: smarter CAR T therapy hits hard to tumors.Front Immunol. 2023 Jul 14;14:1203230. doi: 10.3389/fimmu.2023.1203230. eCollection 2023. Front Immunol. 2023. PMID: 37520522 Free PMC article. Review.
-
Aging-related biomarker discovery in the era of immune checkpoint inhibitors for cancer patients.Front Immunol. 2024 Mar 15;15:1348189. doi: 10.3389/fimmu.2024.1348189. eCollection 2024. Front Immunol. 2024. PMID: 38590525 Free PMC article. Review.
-
Single-cell profiling reveals the intratumor heterogeneity and immunosuppressive microenvironment in cervical adenocarcinoma.Elife. 2025 Mar 11;13:RP97335. doi: 10.7554/eLife.97335. Elife. 2025. PMID: 40066698 Free PMC article.
-
MCTP2 is a novel biomarker promoting tumor progression and nodal metastasis in oral squamous cell carcinoma.Sci Rep. 2025 May 27;15(1):18456. doi: 10.1038/s41598-025-02094-9. Sci Rep. 2025. PMID: 40425609 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

