Pepsin enhances glycolysis to promote malignant transformation of vocal fold leukoplakia epithelial cells with dysplasia
- PMID: 36380093
- PMCID: PMC9988773
- DOI: 10.1007/s00405-022-07729-5
Pepsin enhances glycolysis to promote malignant transformation of vocal fold leukoplakia epithelial cells with dysplasia
Abstract
Purpose: The mechanism underlying malignant transformation of vocal fold leukoplakia (VFL) and the precise role of the expression of pepsin in VFL remain unclear. This study aimed to investigate the effects of acidified pepsin on VFL epithelial cell growth and migration, and also identify pertinent molecular mechanisms.
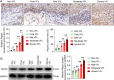
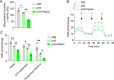
Methods: Immunochemistry and Western blotting were performed to measure glucose transporter type 1 (GLUT1), monocarboxylate transporters 4 (MCT4), and Hexokinase-II (HK-II) expressions. Cell viability, cell cycle, apoptosis, and migration were investigated by CCK-8 assay, flow cytometry and Transwell chamber assay, respectively. Glycolysis-related contents were determined using the corresponding kits. Mitochondrial HK-II was photographed under a confocal microscope using Mito-Tracker Red.
Results: It was found: the expression of pepsin and proportion of pepsin+ cells in VFL increased with the increased dysplasia grade; acidified pepsin enhanced cell growth and migration capabilities of VFL epithelial cells, reduced mitochondrial respiratory chain complex I activity and oxidative phosphorylation, and enhanced aerobic glycolysis and GLUT1 expression in VFL epithelial cells; along with the transfection of GLUT1 overexpression plasmid, 18FFDG uptake, lactate secretion and growth and migration capabilities of VFL epithelial cell were increased; this effect was partially blocked by the glycolysis inhibitor 2-deoxy-glucose; acidified pepsin increased the expression of HK-II and enhanced its distribution in mitochondria of VFL epithelial cells.
Conclusion: It was concluded that acidified pepsin enhances VFL epithelial cell growth and migration abilities by reducing mitochondrial respiratory complex I activity and promoting metabolic reprogramming from oxidative phosphorylation to aerobic glycolysis.
Keywords: GLUT1; Glycolysis; HK-II; Laryngeal cancer; Malignant transformation; Pepsin; Vocal fold leukoplakia.
© 2022. The Author(s).
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

