Preclinical efficacy of azacitidine and venetoclax for infant KMT2A-rearranged acute lymphoblastic leukemia reveals a new therapeutic strategy
- PMID: 36380143
- PMCID: PMC9883157
- DOI: 10.1038/s41375-022-01746-3
Preclinical efficacy of azacitidine and venetoclax for infant KMT2A-rearranged acute lymphoblastic leukemia reveals a new therapeutic strategy
Abstract
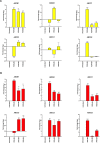
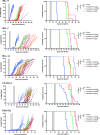
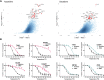
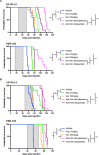
Infants with KMT2A-rearranged B-cell acute lymphoblastic leukemia (ALL) have a dismal prognosis. Survival outcomes have remained static in recent decades despite treatment intensification and novel therapies are urgently required. KMT2A-rearranged infant ALL cells are characterized by an abundance of promoter hypermethylation and exhibit high BCL-2 expression, highlighting potential for therapeutic targeting. Here, we show that hypomethylating agents exhibit in vitro additivity when combined with most conventional chemotherapeutic agents. However, in a subset of samples an antagonistic effect was seen between several agents. This was most evident when hypomethylating agents were combined with methotrexate, with upregulation of ATP-binding cassette transporters identified as a potential mechanism. Single agent treatment with azacitidine and decitabine significantly prolonged in vivo survival in KMT2A-rearranged infant ALL xenografts. Treatment of KMT2A-rearranged infant ALL cell lines with azacitidine and decitabine led to differential genome-wide DNA methylation, changes in gene expression and thermal proteome profiling revealed the target protein-binding landscape of these agents. The selective BCL-2 inhibitor, venetoclax, exhibited in vitro additivity in combination with hypomethylating or conventional chemotherapeutic agents. The addition of venetoclax to azacitidine resulted in a significant in vivo survival advantage indicating the therapeutic potential of this combination to improve outcome for infants with KMT2A-rearranged ALL.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N. Engl J Med. 2015;373:1541–52. - PubMed
-
- Breese EH, Kotecha RS, Guest EM. Acute lymphoblastic leukemia in infants: a distinctive, high-risk subtype of childhood acute lymphoblastic leukemia. In: Litzow MR, Raetz EA (eds). Clinical management of acute lymphoblastic leukemia: from bench to bedside. Springer Nature: Switzerland, 2022, pp 135–48.
-
- Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: results from an international phase III randomized study. J Clin Oncol. 2019;37:2246–56. - PubMed
-
- Stumpel DJ, Schneider P, van Roon EH, Boer JM, de Lorenzo P, Valsecchi MG, et al. Specific promoter methylation identifies different subgroups of MLL-rearranged infant acute lymphoblastic leukemia, influences clinical outcome, and provides therapeutic options. Blood. 2009;114:5490–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

