Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: a randomised controlled trial
- PMID: 36380312
- PMCID: PMC9666938
- DOI: 10.1186/s13054-022-04204-9
Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: a randomised controlled trial
Abstract
Background: Ventilator-associated pneumonia caused by Pseudomonas aeruginosa (PA) in hospitalised patients is associated with high mortality. The effectiveness of the bivalent, bispecific mAb MEDI3902 (gremubamab) in preventing PA nosocomial pneumonia was assessed in PA-colonised mechanically ventilated subjects.
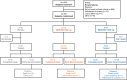
Methods: EVADE (NCT02696902) was a phase 2, randomised, parallel-group, double-blind, placebo-controlled study in Europe, Turkey, Israel, and the USA. Subjects ≥ 18 years old, mechanically ventilated, tracheally colonised with PA, and without new-onset pneumonia, were randomised (1:1:1) to MEDI3902 500, 1500 mg (single intravenous dose), or placebo. The primary efficacy endpoint was the incidence of nosocomial PA pneumonia through 21 days post-dose in MEDI3902 1500 mg versus placebo, determined by an independent adjudication committee.
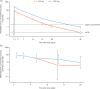
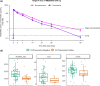
Results: Even if the initial sample size was not reached because of low recruitment, 188 subjects were randomised (MEDI3902 500/1500 mg: n = 16/87; placebo: n = 85) between 13 April 2016 and 17 October 2019. Out of these, 184 were dosed (MEDI3902 500/1500 mg: n = 16/85; placebo: n = 83), comprising the modified intent-to-treat set. Enrolment in the 500 mg arm was discontinued due to pharmacokinetic data demonstrating low MEDI3902 serum concentrations. Subsequently, enrolled subjects were randomised (1:1) to MEDI3902 1500 mg or placebo. PA pneumonia was confirmed in 22.4% (n = 19/85) of MEDI3902 1500 mg recipients and in 18.1% (n = 15/83) of placebo recipients (relative risk reduction [RRR]: - 23.7%; 80% confidence interval [CI] - 83.8%, 16.8%; p = 0.49). At 21 days post-1500 mg dose, the mean (standard deviation) serum MEDI3902 concentration was 9.46 (7.91) μg/mL, with 80.6% (n = 58/72) subjects achieving concentrations > 1.7 μg/mL, a level associated with improved outcome in animal models. Treatment-emergent adverse event incidence was similar between groups.
Conclusions: The bivalent, bispecific monoclonal antibody MEDI3902 (gremubamab) did not reduce PA nosocomial pneumonia incidence in PA-colonised mechanically ventilated subjects. Trial registration Registered on Clinicaltrials.gov ( NCT02696902 ) on 11th February 2016 and on EudraCT ( 2015-001706-34 ) on 7th March 2016.
Keywords: Monoclonal antibody; Pharmacokinetics; Prevention; Pseudomonas aeruginosa ventilator-associated pneumonia; Safety.
© 2022. The Author(s).
Conflict of interest statement
Jean Chastre received personal fees during the conduct of the study from COMBACTE-MAGNET, personal fees from outside of the submitted work from Aridis, Bayer, Inotrem, Shionogi, and Tigenix/Takeda, and grants from AstraZeneca/Medimmune. Bruno François consulted for AM-Pharma, Aridis, Enlivex, GSK, and Inotrem and was part of an adjudication committee for Takeda. Ricard Ferrer received fees for conferences from BD, Grifols, MSD, and Pfizer. Alain Lepape received personal fees outside of the study from Fresenius and Tigenix/Takeda (DSMD committee). Iftihar Koksal has served on advisory boards for Abbvie, MSD, Pfizer, Gilead, GSK, and Roche; lectures: Abbvie, Gilead, Pfizer, and MSD. Charles-Edouard Luyt received fees from Bayer Healthcare, ThermoFisher Brahms, Biomérieux, Faron, Carmat, Aerogen, and Merck Sharp & Dohme outside of the submitted work. Miguel Sánchez-García received funding for speaker fees from Biotest AG, Pfizer, Merck, Sharp & Dohme, AstraZeneca, Orion, and Cepheid; for consulting fees from Bayer, GlaxoSmithKline, Pfizer, and Masimo; for research grants from the European Union, 7th Framework Programme IMI, H2020; was part of an adjudication committee for Takeda. Antoni Torres has served on advisory boards for Pfizer, MSD, Biomérieux, Menarini, Chiesi, and Jansen; lectures: Pfizer and MSD; grants: Bayer, AstraZeneca, and Cardeas. Despoina Koulenti was part of the adjudication committee of EVADE. Thomas L. Holland consulted for Basilea Pharmaceutica, Genentech, and Motif Bio and took part in a Scientific Advisory Board for Motif Bio. Antonio Oliver received grants from and participated as a speaker and in advisory boards for Pfizer, MSD, and Shionogi. Olivier Barraud received speaker fees and/or travel grants from MSD, Pfizer, Roche, and Sanofi and has been a consultant to bioMérieux and Mylan. Herman Goossens received grants from European Union IMI Grant (in collaboration with Novartis). Omar Ali, Ahmad Akhgar, Pin Ren, Terramika Bellamy, Colin Reisner, Alexey Ruzin and Hasan S. Jafri were employees of AstraZeneca during the conduct of the study and hold shares in the company. Kathryn Shoemaker, David E. Tabor, Yuling Wu, Yu Jiang, Antonio DiGiandomenico, Susan Colbert and Mark Esser are employees of AstraZeneca and hold shares in the company. Marc Bourgeois, Apostolos Komnos, Galia Rahav, Nicolas De Schryver, Drieke Vandamme, Surbhi Malhotra-Kumar, Philippe Eggimann, Julien Sauser Frank Coenjaerts, Leen Timbermont, and Marc Bonten have no conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

