Genetic potential for changes in breeding systems: Predicted and observed trait changes during artificial selection for male and female allocation in a gynodioecious species
- PMID: 36380502
- PMCID: PMC9828115
- DOI: 10.1002/ajb2.16096
Genetic potential for changes in breeding systems: Predicted and observed trait changes during artificial selection for male and female allocation in a gynodioecious species
Abstract
Premise: Evolution of separate sexes from hermaphroditism often proceeds through gynodioecy, but genetic constraints on this process are poorly understood. Genetic (co-)variances and between-sex genetic correlations were used to predict evolutionary responses of multiple reproductive traits in a sexually dimorphic gynodioecious species, and predictions were compared with observed responses to artificial selection.
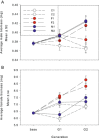
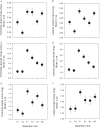
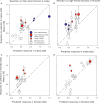
Methods: Schiedea (Caryophyllaceae) is an endemic Hawaiian lineage with hermaphroditic, gynodioecious, subdioecious, and dioecious species. We measured genetic parameters of Schiedea salicaria and used them to predict evolutionary responses of 18 traits in hermaphrodites and females in response to artificial selection for increased male (stamen) biomass in hermaphrodites or increased female (carpel, capsule) biomass in females. Observed responses over two generations were compared with predictions in replicate lines of treatments and controls.
Results: In only two generations, both stamen biomass in hermaphrodites and female biomass in females responded markedly to direct selection, supporting a key assumption of models for evolution of dioecy. Other biomass traits, pollen and ovule numbers, and inflorescence characters important in wind pollination evolved indirectly in response to selection on sex allocation. Responses generally followed predictions from multivariate selection models, with some responses unexpectedly large due to increased genetic correlations as selection proceeded.
Conclusions: Results illustrate the power of artificial selection and utility of multivariate selection models incorporating sex differences. They further indicate that pollen and ovule numbers and inflorescence architecture could evolve in response to selection on biomass allocation to male versus female function, producing complex changes in plant phenotype as separate sexes evolve.
Keywords: Caryophyllaceae; Schiedea; artificial selection; between-sex correlation; dioecy; genetic correlation; inflorescence; sex allocation.
© 2022 The Authors. American Journal of Botany published by Wiley Periodicals LLC on behalf of Botanical Society of America.
Figures




References
-
- Ågren, J. , and Schemske D. W.. 1995. Sex allocation in the monoecious herb Begonia semiovata . Evolution 49: 121–130. - PubMed
-
- Ashman, T. 2003. Constraints on the evolution of dioecy and sexual dimorphism: field estimates of quantitative genetic parameters for reproductive traits in three populations of gynodioecious Fragaria virginiana . Evolution 57: 2012–2025. - PubMed
-
- Campbell, D. R. 1997. Genetic correlation between biomass allocation to male and female functions in a natural plant population. Heredity 79: 606–614.
-
- Campbell, D. R. 2000. Experimental tests of sex‐allocation theory in plants. Trends in Ecology and Evolution 15: 227–232. - PubMed
-
- Campbell, D. R. , Weller S. G., Sakai A. K., Culley T. M., Dang P. N., and Dunbar‐Wallis A. K.. 2010. Genetic variation and covariation in floral allocation of two species of Schiedea with contrasting levels of sexual dimorphism. Evolution 65: 757–770. - PubMed

