Nanotechnology meets immunology towards a rapid diagnosis solution: the COVID-19 outbreak challenge
- PMID: 36380932
- PMCID: PMC9635439
- DOI: 10.1039/d2ra05096j
Nanotechnology meets immunology towards a rapid diagnosis solution: the COVID-19 outbreak challenge
Abstract
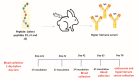
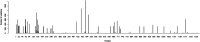
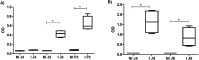
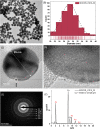
The current COVID-19 pandemic presents one of the greatest challenges in human history. There is a consensus that the rapid and accurate diagnosis of COVID-19 directly affects procedures to avoid dissemination, promote treatments, and favor the prognosis of infected patients. This interdisciplinary study aims at designing new synthetic peptides inspired by the SARS-CoV-2 spike protein (SARS-CoV-2S) to produce rapid detection tests relying on nanomaterial-based colorimetric properties. Hence, in silico analyses of SARS-CoV-2S were performed using advanced bioinformatic simulation tools and algorithms. Five novel peptide sequences were proposed, and three were selected (P2, J4, and J5) based on their prospective reactivity against positive serum from naturally COVID-19-infected humans. Next, hyperimmune sera against the selected peptides were produced in rabbits. Concurrently, gold nanoparticles (AuNP) were synthesized using a green aqueous method under mild conditions through in situ reduction by trisodium citrate salt. They were extensively characterized by their morphological, physicochemical, and optical properties. The AuNPs demonstrated colloidal chemical stability in aqueous media, with an average size of approximately 29 nm (metallic core), and zeta potential before and after bioconjugation of -43 mV and -31 mV, respectively. Moreover, they presented an intense reddish-bluish color due to the surface plasmon resonance (SPR) effect, with maxima at λ = 525 nm and 536 nm, before and after bioconjugation, respectively, evidencing their applicability as colorimetric biomarkers for antigen-antibody immunoassay detection. To develop a rapid COVID-19 diagnosis test using lateral flow assay (LFA), semi-purified anti-SARS-CoV-2S sera against the three selected peptides were bioconjugated to the AuNPs as the highly optically sensitive agents using a considerably low antibody concentration (0.2 mg mL-1). All tested peptide sequences (P2, J4, and J5) induced antibodies capable of identifying the presence of SARS-CoV-2 virus inactivate suspensions (1 : 10, 1 : 100, or 1 : 1000 dilutions). For LFA positive test control, an anti-rabbit antibody was used. In summary, this research comprises several contributions and advances to the broad and multidisciplinary field of nanomaterials-based immunodiagnosis tools, encompassing: (a) the novelty of designing and synthesizing new immunogenic peptides inspired by SARS-CoV-2 virus epitopes using in silico bioinformatics; (b) the peptides induced the immune response in rabbit animal model producing hyperimmune serum; (c) the semi-purified hyperimmune serum rendered effective antibodies to detect SARS-CoV-2 virus in cell suspension; (d) colloidal gold nanoparticles were produced and bioconjugated to the antibodies for qualitative colorimetric detection. As the overall result of this study, it was designed, developed, produced, and validated a new simple, rapid, and sensitive LFA diagnostic test for the SARS-CoV-2 virus using a nanotechnology-based qualitative colorimetric assay, which can be envisioned as promising nanoplatforms for detecting other diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors confirm no competing interests to declare regarding the publication of this article.
Figures







References
-
- WHO, World Health Organization, Weekly epidemiological update on COVID-19, 2021, vol. 69, available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

