Anti-inflammatory and pro-anabolic effects of 5-aminosalicylic acid on human inflammatory osteoarthritis models
- PMID: 36381242
- PMCID: PMC9633873
- DOI: 10.1016/j.jot.2022.10.003
Anti-inflammatory and pro-anabolic effects of 5-aminosalicylic acid on human inflammatory osteoarthritis models
Abstract
Background: Osteoarthritis (OA) is the most common degenerative joint disease, mainly affecting the elderly worldwide, for which the drug treatment remains a major challenge. Low-grade inflammation plays a pivotal role in OA onset and progression. Exploration of notable anti-inflammatory and disease-modifying drugs on human samples could facilitate the evaluation of therapeutic strategies for OA.
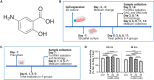
Methods: The anti-inflammatory drug 5-aminosalicylic acid (5-ASA) is a first-line drug for ulcerative colitis (UC), however no study has explored the effects of 5-ASA on articular chondrocytes. In this work, both in vitro (chondrocyte pellets) and ex vivo (osteochondral explants) human inflammatory OA models were applied to evaluate the effects of 5-ASA.
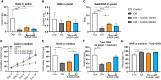
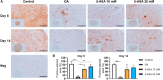
Results: In the inflammatory pellet model, 5-ASA remarkably downregulated the gene expression of interleukin-6 (IL-6), and cyclooxygenase-2 (COX-2) while upregulating proteoglycan 4 (PRG4) and cartilage oligomeric matrix protein (COMP) gene expression. Total glycosaminoglycan (GAG) synthesis by pellets was markedly increased in 5-ASA-treated groups compared with the inflammatory group. In conditioned medium, inflammatory mediators (IL-8, nitric oxide) were markedly inhibited upon 5-ASA treatment. Moreover, histological staining showed 5-ASA retained proteoglycan content and inhibited degradation of extracellular matrix (ECM) core components, aggrecan (ACAN) and collagen type II (COL2). In the inflammatory explant model, 5-ASA mitigated signs of OA development by reducing inflammatory mediators and GAG loss.
Conclusions: These findings suggest that 5-ASA has anti-inflammatory and pro-anabolic effects on human chondrocyte pellet and osteochondral explant inflammatory OA models.
The translational potential of this article: Disease-modifying OA drugs are an unmet clinical need for the treatment of OA. Our study explored and demonstrated the anti-inflammatory and protective effects of 5-ASA on in vitro and ex vivo human inflammatory OA models, showing its translational potential for OA treatment.
Keywords: 5-aminosalicylic acid; Chondrocyte pellet; Inflammatory model; Osteoarthritis; Osteochondral explant; Treatment.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., et al. Osteoarthritis. Nat Rev Dis Prim. 2016;2 - PubMed
-
- Safiri S., Kolahi A.A., Smith E., Hill C., Bettampadi D., Mansournia M.A., et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–828. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

