Association between menstrual cycle length and covid-19 vaccination: global, retrospective cohort study of prospectively collected data
- PMID: 36381261
- PMCID: PMC9665108
- DOI: 10.1136/bmjmed-2022-000297
Association between menstrual cycle length and covid-19 vaccination: global, retrospective cohort study of prospectively collected data
Abstract
Objectives: To identify whether covid-19 vaccines are associated with menstrual changes in order to address concerns about menstrual cycle disruptions after covid-19 vaccination.
Design: Global, retrospective cohort study of prospectively collected data.
Setting: International users of the menstrual cycle tracking application, Natural Cycles.
Participants: 19 622 individuals aged 18-45 years with cycle lengths of 24-38 days and consecutive data for at least three cycles before and one cycle after covid (vaccinated group; n=14 936), and those with at least four consecutive cycles over a similar time period (unvaccinated group; n=4686).
Main outcome measures: The mean change within individuals was assessed by vaccination group for cycle and menses length (mean of three cycles before vaccination to the cycles after first and second dose of vaccine and the subsequent cycle). Mixed effects models were used to estimate the adjusted difference in change in cycle and menses length between the vaccinated and unvaccinated.
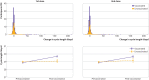
Results: Most people (n=15 713; 80.08%) were younger than 35 years, from the UK (n=6222; 31.71%), US and Canada (28.59%), or Europe (33.55%). Two thirds (9929 (66.48%) of 14 936) of the vaccinated cohort received the Pfizer-BioNTech (BNT162b2) covid-19 vaccine, 17.46% (n=2608) received Moderna (mRNA-1273), 9.06% (n=1353) received Oxford-AstraZeneca (ChAdOx1 nCoV-19), and 1.89% (n=283) received Johnson & Johnson (Ad26.COV2.S). Individuals who were vaccinated had a less than one day adjusted increase in the length of their first and second vaccine cycles, compared with individuals who were not vaccinated (0.71 day increase (99.3% confidence interval 0.47 to 0.96) for first dose; 0.56 day increase (0.28 to 0.84) for second dose). The adjusted difference was larger in people who received two doses in a cycle (3.70 days increase (2.98 to 4.42)). One cycle after vaccination, cycle length was similar to before the vaccine in individuals who received one dose per cycle (0.02 day change (99.3% confidence interval -0.10 to 0.14), but not yet for individuals who received two doses per cycle (0.85 day change (99.3% confidence interval 0.24 to 1.46)) compared with unvaccinated individuals. Changes in cycle length did not differ by the vaccine's mechanism of action (mRNA, adenovirus vector, or inactivated virus). Menses length was unaffected by vaccination.
Conclusions: Covid-19 vaccination is associated with a small and likely to be temporary change in menstrual cycle length but no change in menses length.
Conflict of interest statement
Competing interests AE reports honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, World Health Organization, and Gynuity for committee activities and honorariums for peer review from the Karolinska Institute. AE receives royalties from UptoDate. Oregon Health and Science University (OHSU) receives research funding from OHSU Foundation, Merck, HRA Pharma, and the National Institutes of Health for which Alison Edelman is the principal investigator. BGD reports honorariums and travel reimbursement from the American College of Obstetricians and Society of Family Planning for board, committee, and mentorship activities. OHSU receives research funding from Merck/Organon and Office of Population Affairs/Department of Health and Human Services for which BGD is the principal investigator. OHSU receives research funding from OHSU foundation, the Bill & Melinda Gates Foundation, American Board of Obstetrics and Gynecology, American Society for Reproductive Medicine, and the National Institutes of Health for which Leo Han is the principal investigator. EB, AVL, and JTP are employees of Natural Cycles. Natural Cycles received cost reimbursement from grant funds for data processing and secure transfer. KAM reports honorariums and travel reimbursement from the American Board of Obstetrics and Gynecology and travel reimbursement from American College of Obstetricians and Gynecologists. Women and Infants Hospital received funding from Myovant for consulting work done by KAM on outcomes measures for heavy menstrual bleeding. VM reports research funding from Borne, payment for acting as an external examiner for the Universities of Cambridge, Leeds and Swansea, and royalties received for contribution to Immunology 9th edition (Elsevier). STC is the editor-in-chief of BMJ Sexual and Reproductive Health and reports an honorarium from Gedeon Richter Nordics for a lecture on contraception. ERB declares no competing interests.
Figures

Comment in
-
Does COVID-19 Vaccination Disturb Menstrual Cycling?Am J Epidemiol. 2023 Jun 2;192(6):849-850. doi: 10.1093/aje/kwad039. Am J Epidemiol. 2023. PMID: 36799647
-
Response to "Vaccination and the Menstrual Cycle".Am J Epidemiol. 2023 Jun 2;192(6):851-852. doi: 10.1093/aje/kwad049. Am J Epidemiol. 2023. PMID: 36883904 Free PMC article. No abstract available.
-
Covid-19 vaccines and menstrual changes.BMJ Med. 2022 Oct 21;1(1):e000357. doi: 10.1136/bmjmed-2022-000357. eCollection 2022. BMJ Med. 2022. PMID: 36936587 Free PMC article. No abstract available.
References
-
- Item of interest: NIH funds studies to assess potential effects of COVID-19 vaccination on menstruation. Available: https://www.nichd.nih.gov/.https://www.nichd.nih.gov/newsroom/news/08302... [Accessed 04 Mar 2022].
-
- GOV.UK . COVID-19 vaccines: updates for August 2021. Available: https://www.gov.uk/drug-safety-update/covid-19-vaccines-updates-for-augu... [Accessed 04 Mar 2022].
-
- Trogstad L. Increased occurrence of menstrual disturbances in 18- to 30-year-old women after COVID-19 vaccination. Rochester, NY: Social Science Research Network, 2022. 10.2139/ssrn.3998180 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
