Genitourinary melanoma: An overview for the clinician
- PMID: 36381597
- PMCID: PMC9643129
- DOI: 10.1016/j.ajur.2022.01.003
Genitourinary melanoma: An overview for the clinician
Abstract
Genitourinary (GU) melanoma is a rare presentation of melanoma accounting for approximately 0.5% of all melanomas. GU melanomas include primary melanomas of the vulva, vagina, uterine cervix, ovary, penis, scrotum, urethra, bladder, ureter, and kidney. These melanomas are often diagnosed in advanced stages and stigma is thought to contribute to delays in presentation. As the likely diagnosing provider, it is imperative that dermatologists, urologists, and gynecologists are aware of these uncommon sites of presentation. While there have been major advances in the treatment of melanomas as a whole in the last 10 years, their applications to GU melanomas have often been overlooked. GU melanomas have not been included in many of the major phase III clinical trials which brought contemporary advanced treatments to market and the prognoses for GU melanomas remain poor. Due to the rarity of GU melanomas, much of the literature provides generalized recommendations across multiple different organs affected by GU melanomas or omits certain topics, making it difficult to appreciate the fundamentals of the individual presentations. This review aimed to provide background information on the pathogenesis and epidemiology of the different sites of GU melanomas and categorize data specific to the presentation, staging, treatment, and prognosis of each type of GU melanoma to guide the clinician. It was also meant to encourage a multidisciplinary approach to the management of these patients as it spans the expertise of surgical oncologists, medical oncologists, radiation oncologist, dermatologists, urologists, and gynecologists.
Keywords: Female; Genitalia; Male; Melanoma; Rare diseases; Staging; Treatment; Urinary tract.
© 2022 Editorial Office of Asian Journal of Urology. Production and hosting by Elsevier B.V.
Conflict of interest statement
Jonathan S. Zager has advisory board relationships with Merck, Novartis, Philogen and Castle Biosciences, speaker's bureau for Castle Biosciences, Pfizer, and Sun Pharma. He also receives research funding from Amgen, Delcath Systems, Philogen, Provectus, and Novartis. He serves on the medical advisory board for Delcath Systems. Philippe E. Speiss. serves as the vice chair for the National Comprehensive Cancer Network Panel for bladder and penile cancer. He also serves as an author for UpToDate.com. The remaining authors have no relevant conflicts of interest to disclose.
Figures
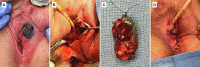
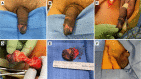
References
-
- Vyas R., Thompson C.L., Zargar H., Selph J., Gerstenblith M.R. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144–150. - PubMed
-
- McLaughlin C.C., Wu X.C., Jemal A., Martin H.J., Roche L.M., Chen V.W. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000–1007. - PubMed
-
- Hussein M.R. Extracutaneous malignant melanomas. Cancer Investig. 2008;26:516–534. - PubMed
-
- Dupin E., Le Douarin N.M. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. - PubMed
-
- Curtin J.A., Busam K., Pinkel D., Bastian B.C. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

