Bacterial extracellular vesicle applications in cancer immunotherapy
- PMID: 36382022
- PMCID: PMC9637733
- DOI: 10.1016/j.bioactmat.2022.10.024
Bacterial extracellular vesicle applications in cancer immunotherapy
Abstract
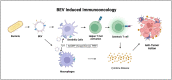
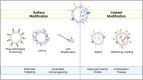
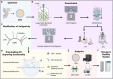
Cancer therapy is undergoing a paradigm shift toward immunotherapy focusing on various approaches to activate the host immune system. As research to identify appropriate immune cells and activate anti-tumor immunity continues to expand, scientists are looking at microbial sources given their inherent ability to elicit an immune response. Bacterial extracellular vesicles (BEVs) are actively studied to control systemic humoral and cellular immune responses instead of using whole microorganisms or other types of extracellular vesicles (EVs). BEVs also provide the opportunity as versatile drug delivery carriers. Unlike mammalian EVs, BEVs have already made it to the clinic with the meningococcal vaccine (Bexsero®). However, there are still many unanswered questions in the use of BEVs, especially for chronic systemically administered immunotherapies. In this review, we address the opportunities and challenges in the use of BEVs for cancer immunotherapy and provide an outlook towards development of BEV products that can ultimately translate to the clinic.
Keywords: Bacterial extracellular vesicles; Cancer immunotherapy; Mammalian extracellular vesicles; Membrane vesicles; Outer membrane vesicles.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Harnessing Bacterial Extracellular Vesicle Immune Effects for Cancer Therapy.Pathog Immun. 2024 Apr 23;9(1):56-90. doi: 10.20411/pai.v9i1.657. eCollection 2024. Pathog Immun. 2024. PMID: 38690563 Free PMC article. Review.
-
Unveiling clinical applications of bacterial extracellular vesicles as natural nanomaterials in disease diagnosis and therapeutics.Acta Biomater. 2024 May;180:18-45. doi: 10.1016/j.actbio.2024.04.022. Epub 2024 Apr 17. Acta Biomater. 2024. PMID: 38641182 Review.
-
Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential.Nano Converg. 2024 Jul 11;11(1):28. doi: 10.1186/s40580-024-00434-5. Nano Converg. 2024. PMID: 38990415 Free PMC article. Review.
-
The emerging role of bacterial extracellular vesicles in human cancers.J Extracell Vesicles. 2024 Oct;13(10):e12521. doi: 10.1002/jev2.12521. J Extracell Vesicles. 2024. PMID: 39377479 Free PMC article. Review.
-
The tremendous biomedical potential of bacterial extracellular vesicles.Trends Biotechnol. 2022 Oct;40(10):1173-1194. doi: 10.1016/j.tibtech.2022.03.005. Epub 2022 May 14. Trends Biotechnol. 2022. PMID: 35581020 Review.
Cited by
-
Bacterial outer membrane vesicles as drug delivery carrier for photodynamic anticancer therapy.Front Chem. 2023 Oct 17;11:1284292. doi: 10.3389/fchem.2023.1284292. eCollection 2023. Front Chem. 2023. PMID: 37915541 Free PMC article. Review.
-
Harnessing Bacterial Extracellular Vesicle Immune Effects for Cancer Therapy.Pathog Immun. 2024 Apr 23;9(1):56-90. doi: 10.20411/pai.v9i1.657. eCollection 2024. Pathog Immun. 2024. PMID: 38690563 Free PMC article. Review.
-
Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure.Int J Mol Sci. 2025 May 23;26(11):5025. doi: 10.3390/ijms26115025. Int J Mol Sci. 2025. PMID: 40507835 Free PMC article. Review.
-
Human Probiotic Lactobacillus paracasei-Derived Extracellular Vesicles Improve Tumor Necrosis Factor-α-Induced Inflammatory Phenotypes in Human Skin.Cells. 2023 Dec 7;12(24):2789. doi: 10.3390/cells12242789. Cells. 2023. PMID: 38132109 Free PMC article.
-
Bacterial Extracellular Vesicles in Oncology: Molecular Mechanisms and Future Clinical Applications.Cancers (Basel). 2025 May 26;17(11):1774. doi: 10.3390/cancers17111774. Cancers (Basel). 2025. PMID: 40507254 Free PMC article. Review.
References
-
- Old L.J. Cancer immunology. Sci. Am. 1977;236(5):62–79. - PubMed
-
- Markovic S.N., Kumar A.B. In: The Basics of Cancer Immunotherapy. Dong H., Markovic S.N., editors. Springer International Publishing; Cham: 2018. Therapeutic targets of FDA-approved immunotherapies in oncology; pp. 21–37.
-
- Young V.B. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

