Harnessing the synergy of perfusable muscle flap matrix and adipose-derived stem cells for prevascularization and macrophage polarization to reconstruct volumetric muscle loss
- PMID: 36382023
- PMCID: PMC9646752
- DOI: 10.1016/j.bioactmat.2022.10.023
Harnessing the synergy of perfusable muscle flap matrix and adipose-derived stem cells for prevascularization and macrophage polarization to reconstruct volumetric muscle loss
Abstract
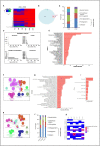
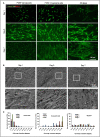
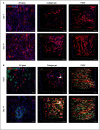
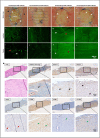
Muscle flaps must have a strong vascular network to support a large tissue volume and ensure successful engraftment. We developed porcine stomach musculofascial flap matrix (PDSF) comprising extracellular matrix (ECM) and intact vasculature. PDSF had a dominant vascular pedicle, microcirculatory vessels, a nerve network, well-retained 3-dimensional (3D) nanofibrous ECM structures, and no allo- or xenoantigenicity. In-depth proteomic analysis demonstrated that PDSF was composed of core matrisome proteins (e.g., collagens, glycoproteins, proteoglycans, and ECM regulators) that, as shown by Gene Ontology term enrichment analysis, are functionally related to musculofascial biological processes. Moreover, PDSF-human adipose-derived stem cell (hASC) synergy not only induced monocytes towards IL-10-producing M2 macrophage polarization through the enhancement of hASCs' paracrine effect but also promoted the proliferation and interconnection of both human skeletal muscle myoblasts (HSMMs) and human umbilical vein endothelial cells (HUVECs) in static triculture conditions. Furthermore, PDSF was successfully prevascularized through a dynamic perfusion coculture of hASCs and HUVECs, which integrated with PDSF and induced the maturation of vascular networks in vitro. In a xenotransplantation model, PDSF demonstrated myoconductive and immunomodulatory properties associated with the predominance of M2 macrophages and regulatory T cells. In a volumetric muscle loss (VML) model, prevascularized PDSF augmented neovascularization and constructive remodeling, which was characterized by the predominant infiltration of M2 macrophages and significant musculofascial tissue formation. These results indicate that hASCs' integration with PDSF enhances the cells' dual function in immunomodulation and angiogenesis. Owing in part to this PDSF-hASC synergy, our platform shows promise for vascularized muscle flap engineering for VML reconstruction.
Keywords: Decellularization; Extracellular matrix; Macrophage polarization; Muscle flap fabrication; Vascularization; Volumetric muscle loss.
© 2022 The Authors.
Figures









Similar articles
-
Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps.Acta Biomater. 2016 Apr 15;35:166-84. doi: 10.1016/j.actbio.2016.02.017. Epub 2016 Feb 12. Acta Biomater. 2016. PMID: 26876876 Free PMC article.
-
Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle.Biomaterials. 2015 Dec;73:198-213. doi: 10.1016/j.biomaterials.2015.09.024. Epub 2015 Sep 18. Biomaterials. 2015. PMID: 26410787 Free PMC article.
-
Decellularized musculofascial extracellular matrix for tissue engineering.Biomaterials. 2013 Apr;34(11):2641-54. doi: 10.1016/j.biomaterials.2012.12.048. Epub 2013 Jan 21. Biomaterials. 2013. PMID: 23347834 Free PMC article.
-
New Insights and Advanced Strategies for In Vitro Construction of Vascularized Tissue Engineering.Tissue Eng Part B Rev. 2023 Dec;29(6):692-709. doi: 10.1089/ten.TEB.2023.0044. Epub 2023 Aug 16. Tissue Eng Part B Rev. 2023. PMID: 37409413 Review.
-
The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration.Regen Ther. 2020 Nov 28;15:285-294. doi: 10.1016/j.reth.2020.11.002. eCollection 2020 Dec. Regen Ther. 2020. PMID: 33426231 Free PMC article. Review.
Cited by
-
Adipose Decellularized Matrix: A Promising Skeletal Muscle Tissue Engineering Material for Volume Muscle Loss.Biomater Res. 2025 Apr 17;29:0174. doi: 10.34133/bmr.0174. eCollection 2025. Biomater Res. 2025. PMID: 40248249 Free PMC article. Review.
-
The research landscape on vascularized composite allografts: a bibliometric analysis (2002-2021).Am J Transl Res. 2023 Mar 15;15(3):1569-1589. eCollection 2023. Am J Transl Res. 2023. PMID: 37056844 Free PMC article.
-
Biomaterial-Based Regenerative Strategies for Volumetric Muscle Loss: Challenges and Solutions.Adv Wound Care (New Rochelle). 2025 Mar;14(3):159-175. doi: 10.1089/wound.2024.0079. Epub 2024 Jul 10. Adv Wound Care (New Rochelle). 2025. PMID: 38775429 Review.
-
Electrospun Silk Fibroin-Silk Sericin Scaffolds Induced Macrophage Polarization and Vascularization for Volumetric Muscle Loss Injury.J Funct Biomater. 2025 Feb 10;16(2):56. doi: 10.3390/jfb16020056. J Funct Biomater. 2025. PMID: 39997590 Free PMC article.
-
Angiogenesis and flap-related research: A bibliometric analysis.Int Wound J. 2023 Oct;20(8):3057-3072. doi: 10.1111/iwj.14181. Epub 2023 Jun 13. Int Wound J. 2023. PMID: 37312275 Free PMC article.
References
-
- Kueckelhaus M., Fischer S., Seyda M., Bueno E.M., Aycart M.A., Alhefz M., ElKhal A., Pomahac B., Tullius S.G. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 2016;29:655–662. doi: 10.1111/tri.12652. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

