Multiscale modelling of claudin-based assemblies: A magnifying glass for novel structures of biological interfaces
- PMID: 36382184
- PMCID: PMC9647347
- DOI: 10.1016/j.csbj.2022.10.038
Multiscale modelling of claudin-based assemblies: A magnifying glass for novel structures of biological interfaces
Abstract
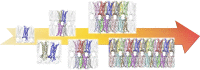
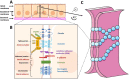
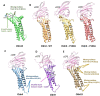
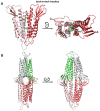
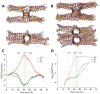
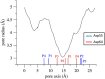
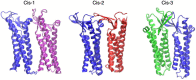
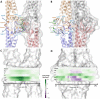
Claudins (Cldns) define a family of transmembrane proteins that are the major determinants of the tight junction integrity and tissue selectivity. They promote the formation of either barriers or ion-selective channels at the interface between two facing cells, across the paracellular space. Multiple Cldn subunits form complexes that include cis- (intracellular) interactions along the membrane of a single cell and trans- (intercellular) interactions across adjacent cells. The first description of Cldn assemblies was provided by electron microscopy, while electrophysiology, mutagenesis and cell biology experiments addressed the functional role of different Cldn homologs. However, the investigation of the molecular details of Cldn subunits and complexes are hampered by the lack of experimental native structures, currently limited to Cldn15. The recent implementation of computer-based techniques greatly contributed to the elucidation of Cldn properties. Molecular dynamics simulations and docking calculations were extensively used to refine the first Cldn multimeric model postulated from the crystal structure of Cldn15, and contributed to the introduction of a novel, alternative, arrangement. While both these multimeric assemblies were found to account for the physiological properties of some family members, they gave conflicting results for others. In this review, we illustrate the major findings on Cldn-based systems that were achieved by using state-of-the-art computational methodologies. The information provided by these results could be useful to improve the characterization of the Cldn properties and help the design of new efficient strategies to control the paracellular transport of drugs or other molecules.
Keywords: APBS, adaptive Poisson-Boltzmann solver; BBB, blood-brain barrier; CAPRI, critical assessment of prediction of interactions; CG, coarse grained; CLDN, claudin; CNS, central nervous system; CV, collective variable; Claudin; Coarse grained molecular dynamics simulations; EA+, ethyl ammonium; ECH, extracellular helix; ECL, extracellular loop; FE, free energy; FFEM, freeze-fracture electron microscopy; FRET, fluorescence resonance energy transfer; Free energy calculations; GPU, graphics processing unit; HB, hydrogen bonds; ICL, intracellular loop; MA+, methylammonium; MD, molecular dynamics; MDCK, Madin-Darby canine kidney; MFPT, mean first passage time; Molecular dynamics simulations; NMR, nuclear magnetic resonance; PAGE, polyacrylamide gel electrophoresis; PANEL, protein association energy landscape; PDB, protein data bank; PMF, potential of mean force; Protein-protein molecular docking; SF, selectivity filter; STED, super-resolution stimulated emission microscopy; TEA+, tetraethylammonium; TEER, transepithelial electric resistance; TEM, transmission electron microscopy; TJ, tight junction; TMA+, tetramethylammonium; Tight junctions; US, umbrella sampling; WT, wild type; X-RD, X-ray diffraction; cCPE, C-terminal fragment of the Clostridium perfringens enterotoxin.
© 2022 The Authors. Published by Elsevier B.V. on behalf of Research Network of Computational and Structural Biotechnology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Zihni C., Mills C., Matter K., et al. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. - PubMed
-
- Meoli L., Günzel D. Channel functions of claudins in the organization of biological systems. Biochim Biophys Acta Biomembr. 2020;1862 - PubMed
-
- Elkouby-Naor L., Ben-Yosef T. Academic Press, International Review of Cell and Molecular Biology; 2010. Chapter 1 – Functions of Claudin Tight Junction Proteins and Their Complex Interactions in Various Physiological Systems; pp. 1–32. - PubMed
-
- Krause G., Protze J., Piontek J. Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin Cell Dev Biol. 2015;42:3–12. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
