A bis-NHC-CAAC dimer derived dicationic diradical
- PMID: 36382295
- PMCID: PMC9629079
- DOI: 10.1039/d2sc03937k
A bis-NHC-CAAC dimer derived dicationic diradical
Abstract
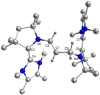
The isolation of carbon-centered diradicals is always challenging due to synthetic difficulties and their limited stability. Herein we report the synthesis of a trans-1,4-cyclohexylene bridged bis-NHC-CAAC dimer derived thermally stable dicationic diradical. The diradical character of this compound was confirmed by EPR spectroscopy. The variable temperature EPR study suggests the singlet state to be marginally more stable than the triplet state (2J = -5.5 cm-1 (ΔE ST = 0.065 kJ mol-1)). The presence of the trans-1,4-cyclohexylene bridge is instrumental for the successful isolation of this dicationic diradical. Notably, in the case of ethylene or propylene bridged bis-NHC-CAAC dimers, the corresponding dicationic diradicals are transient and rearrange to hydrogen abstracted products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
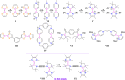
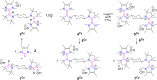


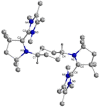

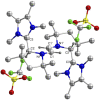

References
-
- Zhang S. Pink M. Junghoefer T. Zhao W. Hsu S. N. Rajca S. Calzolari A. Boudouris B. W. Casu M. B. Rajca A. J. Am. Chem. Soc. 2022;144:6059–6070. doi: 10.1021/jacs.2c01141. - DOI - PMC - PubMed
- Gallagher N. Zhang H. Junghoefer T. Giangrisostomi E. Ovsyannikov R. Pink M. Rajca S. Casu M. B. Rajca A. J. Am. Chem. Soc. 2019;141:4764–4774. doi: 10.1021/jacs.9b00558. - DOI - PMC - PubMed
- Rajca A. Olankitwanit A. Rajca S. J. Am. Chem. Soc. 2011;133:4750–4753. doi: 10.1021/ja200708b. - DOI - PubMed
- Borden W. T. Hoffmann R. Stuyver T. Chen B. J. Am. Chem. Soc. 2017;139:9010–9018. doi: 10.1021/jacs.7b04232. - DOI - PubMed
- Han H. Zhang D. Zhu Z. Wei R. Xiao X. Wang X. Liu Y. Ma Y. Zhao D. J. Am. Chem. Soc. 2021;143:17690–17700. doi: 10.1021/jacs.1c08262. - DOI - PubMed
- Arikawa S. Shimizu A. Shiomi D. Sato K. Shintani R. J. Am. Chem. Soc. 2021;143:19599–19605. doi: 10.1021/jacs.1c10151. - DOI - PubMed
-
- Rajca A. Utamapanya S. Xu J. J. Am. Chem. Soc. 1991;113:9235–9241. doi: 10.1021/ja00024a032. - DOI
- Shu C. Zhang H. Olankitwanit A. Rajca S. Rajca A. J. Am. Chem. Soc. 2019;141:17287–17294. doi: 10.1021/jacs.9b08711. - DOI - PMC - PubMed
- Schmidt D. Son M. Lim J. M. Lin M. J. Krummenacher I. Braunschweig H. Kim D. Würthner F. Angew. Chem., Int. Ed. 2015;54:13980–13984. doi: 10.1002/anie.201507039. - DOI - PubMed
- Rajca A. Shiraishia K. Rajcaa S. Chem. Commun. 2009:4372–4374. doi: 10.1039/B909741D. - DOI - PubMed
-
- Jana A. Ishida M. Park J. S. Bähring S. Jeppesen J. O. Sessler J. L. Chem. Rev. 2017;117:2641–2710. doi: 10.1021/acs.chemrev.6b00375. - DOI - PubMed
- Schröder H. V. Schalley C. A. Beilstein J. Org. Chem. 2018;14:2163–2185. doi: 10.3762/bjoc.14.190. - DOI - PMC - PubMed
- Duvva N. Chilakamarthi U. Giribabu L. Sustainable Energy Fuels. 2017;1:678–688. doi: 10.1039/C7SE00068E. - DOI
-
- Striepe L. Baumgartner T. Chem.–Eur. J. 2017;23:16924–16940. doi: 10.1002/chem.201703348. - DOI - PubMed
- Berville M. Richard J. Stolar M. Choua S. Le Breton N. Gourlaouen C. Boudon C. Ruhlmann L. Baumgartner T. Wytko J. A. Weiss J. Org. Lett. 2018;20:8004–8008. doi: 10.1021/acs.orglett.8b03579. - DOI - PubMed
- Nakazato T. Takekoshi H. Sakurai T. Shinokubo H. Miyake Y. Angew. Chem., Int. Ed. 2021;60:13877–13881. doi: 10.1002/anie.202103667. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

