Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster
- PMID: 36382522
- PMCID: PMC9667363
- DOI: 10.1098/rspb.2022.1642
Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster
Abstract
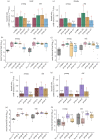
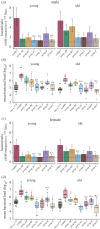
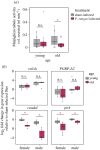
Evolutionary theory predicts a late-life decline in the force of natural selection, possibly leading to late-life deregulations of the immune system. A potential outcome of such deregulations is the inability to produce specific immunity against target pathogens. We tested this possibility by infecting multiple Drosophila melanogaster lines (with bacterial pathogens) across age groups, where either individual or different combinations of Imd- and Toll-inducible antimicrobial peptides (AMPs) were deleted using CRISPR gene editing. We show a high degree of non-redundancy and pathogen-specificity of AMPs in young flies: in some cases, even a single AMP could confer complete resistance. However, ageing led to drastic reductions in such specificity to target pathogens, warranting the action of multiple AMPs across Imd and Toll pathways. Moreover, use of diverse AMPs either lacked survival benefits or even accompanied survival costs post-infection. These features were also sexually dimorphic: females required a larger repertoire of AMPs than males but extracted equivalent survival benefits. Finally, age-specific expansion of the AMP-repertoire was accompanied with ageing-induced downregulation of negative-regulators of the Imd pathway and damage to renal function post-infection, as features of poorly regulated immunity. Overall, we could highlight the potentially non-adaptive role of ageing in producing less-specific AMP responses, across sexes and pathogens.
Keywords: ageing; antimicrobial peptides; immune senescence; immune specificity; pathogen resistance; sexual dimorphism.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

