Cardiac Troponin I-Interacting Kinase Affects Cardiomyocyte S-Phase Activity but Not Cardiomyocyte Proliferation
- PMID: 36382596
- PMCID: PMC9839600
- DOI: 10.1161/CIRCULATIONAHA.122.061130
Cardiac Troponin I-Interacting Kinase Affects Cardiomyocyte S-Phase Activity but Not Cardiomyocyte Proliferation
Abstract
Background: Identifying genetic variants that affect the level of cell cycle reentry and establishing the degree of cell cycle progression in those variants could help guide development of therapeutic interventions aimed at effecting cardiac regeneration. We observed that C57Bl6/NCR (B6N) mice have a marked increase in cardiomyocyte S-phase activity after permanent coronary artery ligation compared with infarcted DBA/2J (D2J) mice.
Methods: Cardiomyocyte cell cycle activity after infarction was monitored in D2J, (D2J×B6N)-F1, and (D2J×B6N)-F1×D2J backcross mice by means of bromodeoxyuridine or 5-ethynyl-2'-deoxyuridine incorporation using a nuclear-localized transgenic reporter to identify cardiomyocyte nuclei. Genome-wide quantitative trait locus analysis, fine scale genetic mapping, whole exome sequencing, and RNA sequencing analyses of the backcross mice were performed to identify the gene responsible for the elevated cardiomyocyte S-phase phenotype.
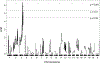
Results: (D2J×B6N)-F1 mice exhibited a 14-fold increase in cardiomyocyte S-phase activity in ventricular regions remote from infarct scar compared with D2J mice (0.798±0.09% versus 0.056±0.004%; P<0.001). Quantitative trait locus analysis of (D2J×B6N)-F1×D2J backcross mice revealed that the gene responsible for differential S-phase activity was located on the distal arm of chromosome 3 (logarithm of the odds score=6.38; P<0.001). Additional genetic and molecular analyses identified 3 potential candidates. Of these, Tnni3k (troponin I-interacting kinase) is expressed in B6N hearts but not in D2J hearts. Transgenic expression of TNNI3K in a D2J genetic background results in elevated cardiomyocyte S-phase activity after injury. Cardiomyocyte S-phase activity in both Tnni3k-expressing and Tnni3k-nonexpressing mice results in the formation of polyploid nuclei.
Conclusions: These data indicate that Tnni3k expression increases the level of cardiomyocyte S-phase activity after injury.
Keywords: cell cycle; myocytes, cardiac; regeneration.
Figures

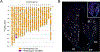



Comment in
-
A Tedious Journey: Cardiomyocyte Proliferation Requires More Than S-Phase Entry and Loss of Polyploidization.Circulation. 2023 Jan 10;147(2):154-157. doi: 10.1161/CIRCULATIONAHA.122.062784. Epub 2023 Jan 9. Circulation. 2023. PMID: 36622907 No abstract available.
References
-
- Rumyantsev PP. Growth and hyperplasia of cardiac muscle cells. London, U.K.; New York, N.Y., U.S.A.: Harwood Academic Publishers; 1991.
-
- Li F, Wang X, Capasso JM and Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–46. - PubMed
-
- Soonpaa MH, Zebrowski DC, Platt C, Rosenzweig A, Engel FB and Field LJ. Cardiomyocyte cell-cycle activity during preadolescence. Cell. 2015;163:781–2. - PubMed
-
- Soonpaa MH, Kim KK, Pajak L, Franklin M and Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–9. - PubMed
-
- Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL and Bergmann O. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell. 2015;163:1026–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

