The role of extracellular histones in COVID-19
- PMID: 36382685
- PMCID: PMC10108027
- DOI: 10.1111/joim.13585
The role of extracellular histones in COVID-19
Abstract
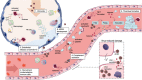
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had spread from China and, within 2 months, became a global pandemic. The infection from this disease can cause a diversity of symptoms ranging from asymptomatic to severe acute respiratory distress syndrome with an increased risk of vascular hyperpermeability, pulmonary inflammation, extensive lung damage, and thrombosis. One of the host defense systems against coronavirus disease 2019 (COVID-19) is the formation of neutrophil extracellular traps (NETs). Numerous studies on this disease have revealed the presence of elevated levels of NET components, such as cell-free DNA, extracellular histones, neutrophil elastase, and myeloperoxidase, in plasma, serum, and tracheal aspirates of severe COVID-19 patients. Extracellular histones, a major component of NETs, are clinically very relevant as they represent promising biomarkers and drug targets, given that several studies have identified histones as key mediators in the onset and progression of various diseases, including COVID-19. However, the role of extracellular histones in COVID-19 per se remains relatively underexplored. Histones are nuclear proteins that can be released into the extracellular space via apoptosis, necrosis, or NET formation and are then regarded as cytotoxic damage-associated molecular patterns that have the potential to damage tissues and impair organ function. This review will highlight the mechanisms of extracellular histone-mediated cytotoxicity and focus on the role that histones play in COVID-19. Thereby, this paper facilitates a bench-to-bedside view of extracellular histone-mediated cytotoxicity, its role in COVID-19, and histones as potential drug targets and biomarkers for future theranostics in the clinical treatment of COVID-19 patients.
Keywords: COVID-19; DAMPs; NETosis; NETs; extracellular histones; histones.
© 2022 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
CR and GN are inventors of a patent owned by the Maastricht University on the use of non‐anticoagulant heparin to treat histone‐mediated inflammatory disease. KW and GN are inventors of a patent filed by the Maastricht University to use peptides to target extracellular histones H4 and H2A.
Figures


References
-
- World Health Organisation 2013 . WHO Coronavirus (COVID‐19) Dashboard . The United Nations, https://covid19.who.int. Accessed 30 November 2022
-
- Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

