Binary and analog variation of synapses between cortical pyramidal neurons
- PMID: 36382887
- PMCID: PMC9704804
- DOI: 10.7554/eLife.76120
Binary and analog variation of synapses between cortical pyramidal neurons
Abstract
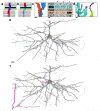
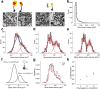
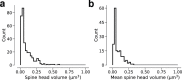
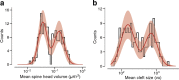
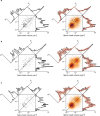
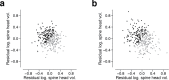
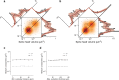
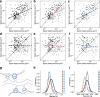
Learning from experience depends at least in part on changes in neuronal connections. We present the largest map of connectivity to date between cortical neurons of a defined type (layer 2/3 [L2/3] pyramidal cells in mouse primary visual cortex), which was enabled by automated analysis of serial section electron microscopy images with improved handling of image defects (250 × 140 × 90 μm3 volume). We used the map to identify constraints on the learning algorithms employed by the cortex. Previous cortical studies modeled a continuum of synapse sizes by a log-normal distribution. A continuum is consistent with most neural network models of learning, in which synaptic strength is a continuously graded analog variable. Here, we show that synapse size, when restricted to synapses between L2/3 pyramidal cells, is well modeled by the sum of a binary variable and an analog variable drawn from a log-normal distribution. Two synapses sharing the same presynaptic and postsynaptic cells are known to be correlated in size. We show that the binary variables of the two synapses are highly correlated, while the analog variables are not. Binary variation could be the outcome of a Hebbian or other synaptic plasticity rule depending on activity signals that are relatively uniform across neuronal arbors, while analog variation may be dominated by other influences such as spontaneous dynamical fluctuations. We discuss the implications for the longstanding hypothesis that activity-dependent plasticity switches synapses between bistable states.
Keywords: connectivity diagram; electron microscopy; mouse; neuroscience; pyramidal cell; synapses.
© 2022, Dorkenwald, Turner, Macrina et al.
Conflict of interest statement
SD, NT, KL, RL, JW, AB, AB, DB, NK, WS, DI, JZ, AZ, IT, SY, SP, WW, MC, CJ, AW, EF, JB, MT, RT, GM, FC, CS, DB, YL, LB, SS, NM, RR No competing interests declared, TM, HS discloses financial interests in Zetta AI LLC, JR, AT discloses financial interests in Vathes LLC
Figures









References
-
- Amit DJ, Fusi S. Learning in neural networks with material synapses. Neural Computation. 1994;6:957–982. doi: 10.1162/neco.1994.6.5.957. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

