Prediction Model for Contractile Function of Circulatory Death Donor Hearts Based on Microvascular Flow Shifts During Ex Situ Hypothermic Cardioplegic Machine Perfusion
- PMID: 36382941
- PMCID: PMC9851462
- DOI: 10.1161/JAHA.122.027146
Prediction Model for Contractile Function of Circulatory Death Donor Hearts Based on Microvascular Flow Shifts During Ex Situ Hypothermic Cardioplegic Machine Perfusion
Abstract
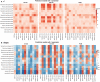
Background Hearts procured from circulatory death donors (DCD) are predominantly maintained by machine perfusion (MP) with normothermic donor blood. Currently, DCD heart function is evaluated by lactate and visual inspection. We have shown that MP with the cardioplegic, crystalloid Custodiol-N solution is superior to blood perfusion to maintain porcine DCD hearts. However, no method has been developed yet to predict the contractility of DCD hearts after cardioplegic MP. We hypothesize that the shift of microvascular flow during continuous MP with a cardioplegic preservation solution predicts the contractility of DCD hearts. Methods and Results In a pig model, DCD hearts were harvested and maintained by MP with hypothermic, oxygenated Custodiol-N for 4 hours while myocardial microvascular flow was measured by Laser Doppler Flow (LDF) technology. Subsequently, hearts were perfused with blood for 2 hours, and left ventricular contractility was measured after 30 and 120 minutes. Various novel parameters which represent the LDF shift were computed. We used 2 combined LDF shift parameters to identify bivariate prediction models. Using the new prediction models based on LDF shifts, highest r2 for end-systolic pressure was 0.77 (P=0.027), for maximal slope of pressure increment was 0.73 (P=0.037), and for maximal slope of pressure decrement was 0.75 (P=0.032) after 30 minutes of reperfusion. After 120 minutes of reperfusion, highest r2 for end-systolic pressure was 0.81 (P=0.016), for maximal slope of pressure increment was 0.90 (P=0.004), and for maximal slope of pressure decrement was 0.58 (P=0.115). Identical prediction models were identified for maximal slope of pressure increment and for maximal slope of pressure decrement at both time points. Lactate remained constant and therefore was unsuitable for prediction. Conclusions Contractility of DCD hearts after continuous MP with a cardioplegic preservation solution can be predicted by the shift of LDF during MP.
Keywords: Custodiol‐N; coronary microvasculature; donation after circulatory death; heart transplantation; machine perfusion; myocardial microcirculation; prediction model.
Figures





References
-
- Messer S, Cernic S, Page A, Berman M, Kaul P, Colah S, Ali J, Pavlushkov E, Baxter J, Quigley R, et al. A 5‐year single‐center early experience of heart transplantation from donation after circulatory‐determined death donors. J Heart Lung Transplant. 2020;39:1463–1475. doi: 10.1016/j.healun.2020.10.001 - DOI - PubMed
-
- Tchana‐Sato V, Ledoux D, Detry O, Hans G, Ancion A, D'Orio V, Massion PB, Amabili P, Bruls S, Lavigne JP, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38:593–598. doi: 10.1016/j.healun.2019.02.015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

