Biosensing Tacrolimus in Human Whole Blood by Using a Drug Receptor Fused to the Emerald Green Fluorescent Protein
- PMID: 36382944
- PMCID: PMC9716524
- DOI: 10.1021/acs.analchem.2c03122
Biosensing Tacrolimus in Human Whole Blood by Using a Drug Receptor Fused to the Emerald Green Fluorescent Protein
Abstract
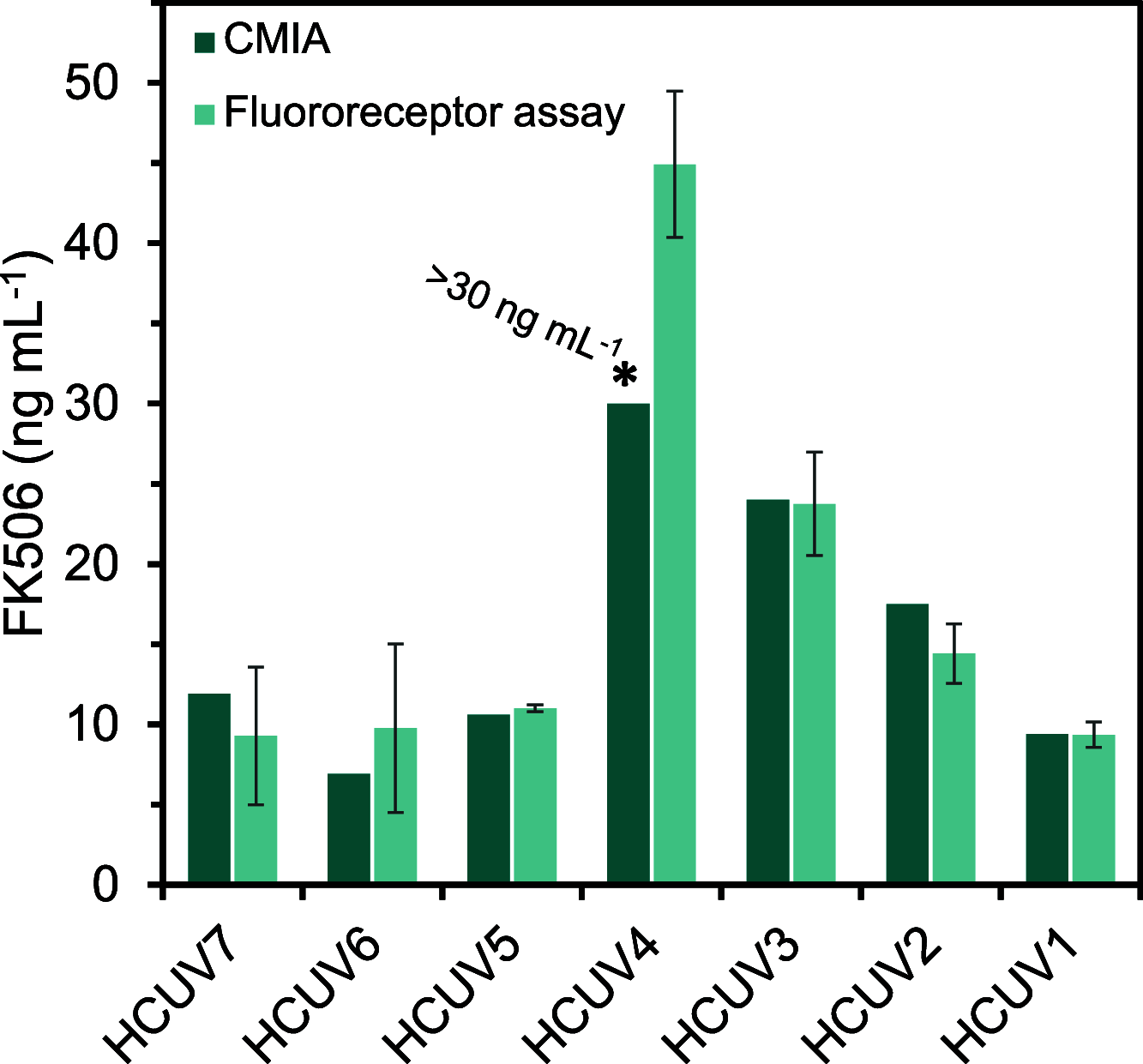
Tacrolimus (FK506) is an immunosuppressant drug (ISD) used to prevent organ rejection after transplantation that exhibits a narrow therapeutic window and is subject to wide inter- and intra-individual pharmacokinetic fluctuations requiring careful monitoring. The immunosuppressive capacity of FK506 arises from the formation of a complex with immunophilin FKBP1A. This paper describes the use of FKBP1A as an alternative to common antibodies for biosensing purposes. Bioassays use recombinant FKBP1A fused to the emerald green fluorescent protein (FKBP1A-EmGFP). Samples containing the immunosuppressant are incubated with the recombinant protein, and free FKBP1A-EmGFP is captured by magnetic beads functionalized with FK506 to generate a fluorescence signal. Recombinant receptor-drug interaction is evaluated by using a quartz crystal microbalance and nuclear magnetic resonance. The limit of detection (3 ng mL-1) and dynamic range thus obtained (5-70 ng mL-1) fulfill therapeutic requirements. The assay is selective for other ISD usually coadministered with FK506 and allows the drug to be determined in human whole blood samples from organ transplant patients with results comparing favorably with those of an external laboratory.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Tanaka H.; Kuroda A.; Marusawa H.; Hatanaka H.; Kino T.; Goto T.; Hashimoto M.; Taga T. Structure of FK506, a Novel Immunosuppressant Isolated from Streptomyces. J. Am. Chem. Soc. 1987, 109, 5031–5033. 10.1021/ja00250a050. - DOI
-
- Personalized Immunosuppression in Transplantation: Role of Biomarker Monitoring and Therapeutic Drug Monitoring; Oellerich M., Dasgupta A., Eds.; Elsevier: Amsterdam, 2016.
-
- Wallemacq P.; Armstrong V. W.; Brunet M.; Haufroid V.; Holt D. W.; Johnston A.; Kuypers D.; Meur Y. L.; Marquet P.; Oellerich M.; Thervet E.; Toenshoff B.; Undre N.; Weber L. T.; Westley I. S.; Mourad M. Opportunities to Optimize Tacrolimus Therapy in Solid Organ Transplantation: Report of the European Consensus Conference. Ther. Drug Monit. 2009, 31, 139–152. 10.1097/ftd.0b013e318198d092. - DOI - PubMed
-
- Udomkarnjananun S.; Francke M. I.; De Winter B. C. M.; Mulder M. B.; Baan C. C.; Metselaar H. J.; den Hoed C. M.; Hesselink D. A. Therapeutic Drug Monitoring of Immunosuppressive Drugs in Hepatology and Gastroenterology. Best Pract. Res. Clin. Gastroenterol. 2021, 54-55, 101756.10.1016/j.bpg.2021.101756. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

