Contraceptives and cancer risks in BRCA1/2 pathogenic variant carriers: a systematic review and meta-analysis
- PMID: 36383189
- PMCID: PMC9976973
- DOI: 10.1093/humupd/dmac038
Contraceptives and cancer risks in BRCA1/2 pathogenic variant carriers: a systematic review and meta-analysis
Abstract
Background: Increasing numbers of BReast CAncer (BRCA) 1 or 2 pathogenic variant (PV) carriers, who have an inherited predisposition to breast and ovarian cancer, are being identified. Among these women, data regarding the effects of contraception on cancer risks are unclear and various guidelines provide various recommendations.
Objective and rationale: We aim to optimize counselling regarding contraception for BRCA1/2-PV carriers. Therefore, we performed a systematic review and meta-analysis. We investigated the risk ratio for developing breast cancer or ovarian cancer in BRCA1/2-PV carriers who have used any form of contraception versus non-users. Second, we analysed breast and ovarian cancer risk among BRCA1/2-PV carriers as influenced by the duration of contraceptive use and by the time since last use. In addition, we provide an overview of all relevant international guidelines regarding contraceptive use for BRCA1/2-PV carriers.
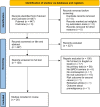
Search methods: A systematic search in the Medline database and Cochrane library identified studies describing breast and/or ovarian cancer risk in BRCA1/2-PV carriers as modified by contraception until June 2021. The search included medical subject headings, keywords and synonyms related to BRCA and contraceptives (any kind). PRISMA guidance was followed. Risk Of Bias In Non-randomized Studies of Interventions and Grading of Recommendations, Assessment, Development and Evaluations assessments were performed. Random-effects meta-analyses were used to estimate pooled effects for breast and ovarian cancer risk separately. Subgroup analyses were conducted for BRCA1 versus BRCA2 and for the various contraceptive methods.
Outcomes: Results of the breast cancer risk with oral contraceptive pill (OCP) analysis depended on the outcome measure. Meta-analyses of seven studies with 7525 women revealed a hazard ratio (HR) of 1.55 (95% CI: 1.36-1.76) and of four studies including 9106 women resulted in an odds ratio (OR) of 1.06 (95% CI: 0.90-1.25), heterogeneity (I2) 0% and 52%, respectively. Breast cancer risk was still increased in ever-users compared with never-users >10 years after last OCP use. In contrast, ovarian cancer risk was decreased among OCP users: HR 0.62 (95% CI: 0.52-0.74) based on two studies including 10 981 women (I2: 0%), and OR 0.49 (95% CI: 0.38-0.63) based on eight studies including 10 390 women (I2: 64%). The protective effect vanished after cessation of use. Tubal ligation also protects against ovarian cancer: one study including 3319 women (I2: 0%): HR: 0.44 (95% CI: 0.26-0.74) and three studies with 7691 women (I2: 44%): OR: 0.74 (95% CI: 0.53-1.03). Data regarding other contraceptives were unavailable. No differences were observed between BRCA1 and BRCA2-PV carriers. The quality of evidence was either low or very low.
Wider implications: The OCP potentially increases breast cancer risk, while ovarian cancer risk decreases with either the OCP and tubal ligation in BRCA1/2-PV carriers. Counselling of BRCA1/2-PV carriers should be personalized; the genetic and non-genetic factors (like prior risk-reducing surgeries, prior breast cancer and age) and patients' preferences (reversibility, ease of use, reliability and effect on menstrual cycle) should be balanced. To further optimize counselling for high-risk women, future research should focus on other (commonly used) contraceptive methods and cancer risks in this specific population, and on the potential impact of changing formulations over time.
Keywords: BRCA1 gene; BRCA2 gene; breast cancer; contraception; oral contraceptive pill; ovarian cancer; tubal ligation.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Antoniou AC, Rookus M, Andrieu N, Brohet R, Chang-Claude J, Peock S, Cook M, Evans DG, Eeles R, Nogues C et al; GEO-HEBON. Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev 2009;18:601–610. - PubMed
-
- Balayla J, Gil Y, Lasry A, Mitric C.. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol 2021;41:848–853. - PubMed
-
- Barnhoorn PC, Bruinsma ACA, Bouma M, Damen Z, De Swart SM, Koetsier MJE, Kurver MJ, Van der Sande R, Van der Wijden CL, Van Groeningen COM;. NHG-standaard Anticonceptie. Utrecht: Nederlands Huisartsen Genootschap (NHG) (Dutch Society of General Practitioners), 2020. https://richtlijnen.nhg.org/standaarden/anticonceptie (1 June 2021, date last accessed).
-
- Beral V, Doll R, Hermon C, Peto R, Reeves G;. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008;371:303–314. - PubMed
-
- Bernholtz S, Laitman Y, Kaufman B, Paluch Shimon S, Friedman E.. Cancer risk in Jewish BRCA1 and BRCA2 mutation carriers: effects of oral contraceptive use and parental origin of mutation. Breast Cancer Res Treat 2011;129:557–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

