Conversion of CO2 into organic acids by engineered autotrophic yeast
- PMID: 36383601
- PMCID: PMC9704707
- DOI: 10.1073/pnas.2211827119
Conversion of CO2 into organic acids by engineered autotrophic yeast
Abstract
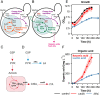
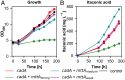
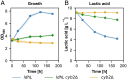
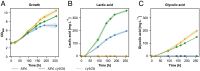
The increase of CO2 emissions due to human activity is one of the preeminent reasons for the present climate crisis. In addition, considering the increasing demand for renewable resources, the upcycling of CO2 as a feedstock gains an extensive importance to establish CO2-neutral or CO2-negative industrial processes independent of agricultural resources. Here we assess whether synthetic autotrophic Komagataella phaffii (Pichia pastoris) can be used as a platform for value-added chemicals using CO2 as a feedstock by integrating the heterologous genes for lactic and itaconic acid synthesis. 13C labeling experiments proved that the resulting strains are able to produce organic acids via the assimilation of CO2 as a sole carbon source. Further engineering attempts to prevent the lactic acid consumption increased the titers to 600 mg L-1, while balancing the expression of key genes and modifying screening conditions led to 2 g L-1 itaconic acid. Bioreactor cultivations suggest that a fine-tuning on CO2 uptake and oxygen demand of the cells is essential to reach a higher productivity. We believe that through further metabolic and process engineering, the resulting engineered strain can become a promising host for the production of value-added bulk chemicals by microbial assimilation of CO2, to support sustainability of industrial bioprocesses.
Keywords: carbon capture; metabolic engineering; organic acids; synthetic biology; yeast.
Conflict of interest statement
D.M. and T.G. are co-inventors of a patent application disclosing organic acid production with synthetic autotrophic yeasts.
Figures
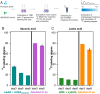
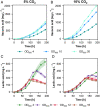

References
-
- Friedlingstein P., et al. , Global carbon budget 2021. Earth Syst. Sci. Data 14, 1917–2005 (2022).
-
- Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P., Primary production of the biosphere: Integrating terrestrial and oceanic components. Science (80-.) 281, 237–240 (1998). - PubMed
-
- Kirst H., Formighieri C., Melis A., Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim. Biophys. Acta 1837, 1653–1664 (2014). - PubMed
-
- Liu C., Colón B. C., Ziesack M., Silver P. A., Nocera D. G., Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science (80-.) 352, 1210–1213 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

