CTR9 drives osteochondral lineage differentiation of human mesenchymal stem cells via epigenetic regulation of BMP-2 signaling
- PMID: 36383652
- PMCID: PMC9668309
- DOI: 10.1126/sciadv.adc9222
CTR9 drives osteochondral lineage differentiation of human mesenchymal stem cells via epigenetic regulation of BMP-2 signaling
Abstract
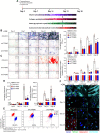
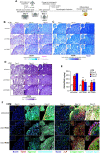
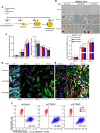
Cell fate determination of human mesenchymal stem/stromal cells (hMSCs) is precisely regulated by lineage-specific transcription factors and epigenetic enzymes. We found that CTR9, a key scaffold subunit of polymerase-associated factor complex (PAFc), selectively regulates hMSC differentiation to osteoblasts and chondrocytes, but not to adipocytes. An in vivo ectopic osteogenesis assay confirmed the essentiality of CTR9 in hMSC-derived bone formation. CTR9 counteracts the activity of Enhancer Of Zeste 2 (EZH2), the epigenetic enzyme that deposits H3K27me3, in hMSCs. Accordingly, CTR9 knockdown (KD) hMSCs gain H3K27me3 mark, and the osteogenic differentiation defects of CTR9 KD hMSCs can be partially rescued by treatment with EZH2 inhibitors. Transcriptome analyses identified bone morphology protein-2 (BMP-2) as a downstream effector of CTR9. BMP-2 secretion, membrane anchorage, and the BMP-SMAD pathway were impaired in CTR9 KD MSCs, and the effects were rescued by BMP-2 supplementation. This study uncovers an epigenetic mechanism engaging the CTR9-H3K27me3-BMP-2 axis to regulate the osteochondral lineage differentiation of hMSCs.
Figures





References
-
- L. A. Marquez-Curtis, A. Janowska-Wieczorek, L. E. McGann, J. A. Elliott,Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 71,181–197 (2015). - PubMed
-
- T. Hoshiba, N. Kawazoe, G. Chen,The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 33,2025–2031 (2012). - PubMed
-
- I. Shur, O. Reish, E. Ezra, D. Benayahu,Analysis of mesenchymal cells derived from an chondrodysplasia punctuate patient and donors. J. Cell. Biochem. 93,112–119 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

