Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment
- PMID: 36383683
- PMCID: PMC10028729
- DOI: 10.1126/scitranslmed.abo3445
Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment
Abstract
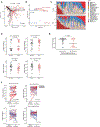
Not all patients with cancer and severe neutropenia develop fever, and the fecal microbiome may play a role. In a single-center study of patients undergoing hematopoietic cell transplant (n = 119), the fecal microbiome was characterized at onset of severe neutropenia. A total of 63 patients (53%) developed a subsequent fever, and their fecal microbiome displayed increased relative abundances of Akkermansia muciniphila, a species of mucin-degrading bacteria (P = 0.006, corrected for multiple comparisons). Two therapies that induce neutropenia, irradiation and melphalan, similarly expanded A. muciniphila and additionally thinned the colonic mucus layer in mice. Caloric restriction of unirradiated mice also expanded A. muciniphila and thinned the colonic mucus layer. Antibiotic treatment to eradicate A. muciniphila before caloric restriction preserved colonic mucus, whereas A. muciniphila reintroduction restored mucus thinning. Caloric restriction of unirradiated mice raised colonic luminal pH and reduced acetate, propionate, and butyrate. Culturing A. muciniphila in vitro with propionate reduced utilization of mucin as well as of fucose. Treating irradiated mice with an antibiotic targeting A. muciniphila or propionate preserved the mucus layer, suppressed translocation of flagellin, reduced inflammatory cytokines in the colon, and improved thermoregulation. These results suggest that diet, metabolites, and colonic mucus link the microbiome to neutropenic fever and may guide future microbiome-based preventive strategies.
Figures


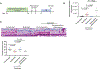



References
-
- Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA, Nastoupil LJ, Rajotte M, Rolston KV, Strasfeld L, Flowers CR, Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol 36, 3043–3054 (2018); published online EpubOct 20 (10.1200/JCO.18.00374). - DOI - PubMed
-
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious A Diseases Society of, Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 52, e56–93 (2011); published online EpubFeb 15 (10.1093/cid/cir073). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

