Protein kinase Cι mediates immunosuppression in lung adenocarcinoma
- PMID: 36383684
- PMCID: PMC11457891
- DOI: 10.1126/scitranslmed.abq5931
Protein kinase Cι mediates immunosuppression in lung adenocarcinoma
Abstract
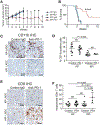
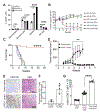
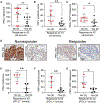
Lung adenocarcinoma (LUAD) is the most prevalent form of non-small cell lung cancer (NSCLC) and a leading cause of cancer death. Immune checkpoint inhibitors (ICIs) of programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) signaling induce tumor regressions in a subset of LUAD, but many LUAD tumors exhibit resistance to ICI therapy. Here, we identified Prkci as a major determinant of response to ICI in a syngeneic mouse model of oncogenic mutant Kras/Trp53 loss (KP)-driven LUAD. Protein kinase Cι (PKCι)-dependent KP tumors exhibited resistance to anti-PD-1 antibody therapy (α-PD-1), whereas KP tumors in which Prkci was genetically deleted (KPI tumors) were highly responsive. Prkci-dependent resistance to α-PD-1 was characterized by enhanced infiltration of myeloid-derived suppressor cells (MDSCs) and decreased infiltration of CD8+ T cells in response to α-PD-1. Mechanistically, Prkci regulated YAP1-dependent expression of Cxcl5, which served to attract MDSCs to KP tumors. The PKCι inhibitor auranofin inhibited KP tumor growth and sensitized these tumors to α-PD-1, whereas expression of either Prkci or its downstream effector Cxcl5 in KPI tumors induced intratumoral infiltration of MDSCs and resistance to α-PD-1. PRKCI expression in tumors of patients with LUAD correlated with genomic signatures indicative of high YAP1-mediated transcription, elevated MDSC infiltration and low CD8+ T cell infiltration, and with elevated CXCL5/6 expression. Last, PKCι-YAP1 signaling was a biomarker associated with poor response to ICI in patients with LUAD. Our data indicate that immunosuppressive PKCι-YAP1-CXCL5 signaling is a key determinant of response to ICI, and pharmacologic inhibition of PKCι may improve therapeutic response to ICI in patients with LUAD.
Conflict of interest statement
Figures




References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2021. CA Cancer J. Clin 71, 7–33 (2021). - PubMed
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions, The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol 11, 39–51 (2016). - PubMed
-
- Dafni U, Tsourti Z, Vervita K, Peters S, Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 134, 127–140 (2019). - PubMed
-
- Yamauchi Y, Safi S, Blattner C, Rathinasamy A, Umansky L, Juenger S, Warth A, Eichhorn M, Muley T, Herth FJF, Dienemann H, Platten M, Beckhove P, Utikal J, Hoffmann H, Umansky V, Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am. J. Respir. Crit. Care Med 198, 777–787 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

