Pathogen spillover driven by rapid changes in bat ecology
- PMID: 36384167
- PMCID: PMC9768785
- DOI: 10.1038/s41586-022-05506-2
Pathogen spillover driven by rapid changes in bat ecology
Abstract
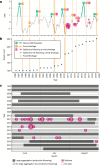
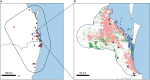
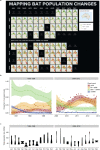
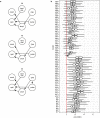
During recent decades, pathogens that originated in bats have become an increasing public health concern. A major challenge is to identify how those pathogens spill over into human populations to generate a pandemic threat1. Many correlational studies associate spillover with changes in land use or other anthropogenic stressors2,3, although the mechanisms underlying the observed correlations have not been identified4. One limitation is the lack of spatially and temporally explicit data on multiple spillovers, and on the connections among spillovers, reservoir host ecology and behaviour and viral dynamics. We present 25 years of data on land-use change, bat behaviour and spillover of Hendra virus from Pteropodid bats to horses in subtropical Australia. These data show that bats are responding to environmental change by persistently adopting behaviours that were previously transient responses to nutritional stress. Interactions between land-use change and climate now lead to persistent bat residency in agricultural areas, where periodic food shortages drive clusters of spillovers. Pulses of winter flowering of trees in remnant forests appeared to prevent spillover. We developed integrative Bayesian network models based on these phenomena that accurately predicted the presence or absence of clusters of spillovers in each of the 25 years. Our long-term study identifies the mechanistic connections between habitat loss, climate and increased spillover risk. It provides a framework for examining causes of bat virus spillover and for developing ecological countermeasures to prevent pandemics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Comment in
-
Why do bat viruses keep infecting people?Nature. 2022 Nov;611(7937):646-647. doi: 10.1038/d41586-022-03682-9. Nature. 2022. PMID: 36385292 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

