Endothelin A receptors contribute to senescence of brain microvascular endothelial cells
- PMID: 36384316
- PMCID: PMC10052805
- DOI: 10.1139/cjpp-2022-0071
Endothelin A receptors contribute to senescence of brain microvascular endothelial cells
Abstract
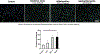
Cellular senescence plays a pivotal role in the aging and progression of neurodegenerative diseases, including vascular cognitive impairment and dementia (VCID). In postmortem brains from individuals with VCID, endothelin-1 (ET-1) levels closely correlate with blood barrier breakdown and cerebral hypoperfusion. Brain microvascular endothelial cells (BMVECs), previously thought to have exclusively endothelin B receptors, also possess endothelin A (ETA) receptors; however, the functional significance of this receptor in BMVECs is not known. We hypothesize that ETA receptors mediate BMVEC senescence. Serum-starved human BMVECs (HBEC5i) were incubated with ET-1 (1 µmol/L) in the presence/absence of ETA receptor antagonist BQ-123 (20 µmol/L). Cells were collected for Western blot and quantitative real-time PCR analyses. Treatment of ET-1 increased protein expression of ETA receptor, while it was prevented by the ETA receptor antagonist. ET-1 increased p21, p16, p53, LIF1 and cyclin D1 protein levels, and β-galactosidase accumulation, which were prevented in the presence of ETA blockade. While there was no change in tight junction proteins, ET-1 decreased adherent junction protein vascular endothelial cadherin (VE-cadherin) levels. In conclusion, ET-1 upregulates ETA receptors in BMVECs in an autocrine manner and triggers the activation of senescence. These in vitro findings need to be further studied in vivo to establish the role of ETA receptors in the progression of endothelial senescence in VCID.
Keywords: brain; cellules endothéliales; cerveau; endothelial cells; endothelin-1; endothéline 1; senescence; sénescence.
Conflict of interest statement
COMPETING INTEREST
The authors declare there are no funding conflicts or competing interests.
Figures

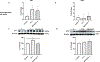

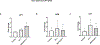
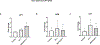
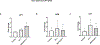

References
-
- Alcalde-Estevez E, Asenjo-Bueno A, Sosa P, Olmos G, Plaza P, Caballero-Mora MA, Rodriguez-Puyol D, Ruiz-Torres MP, and Lopez-Ongil S 2020. Endothelin-1 induces cellular senescence and fibrosis in cultured myoblasts. A potential mechanism of aging-related sarcopenia. Aging (Albany NY) 12(12): 11200–11223. DOI: 10.18632/aging.103450. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

