Building bridges: mycelium-mediated plant-plant electrophysiological communication
- PMID: 36384396
- PMCID: PMC9673936
- DOI: 10.1080/15592324.2022.2129291
Building bridges: mycelium-mediated plant-plant electrophysiological communication
Abstract
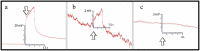
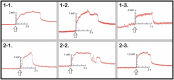
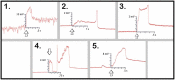
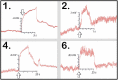
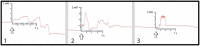
Whether through root secretions or by emitting volatile organic compounds, plant communication has been well-documented. While electrical activity has been documented in plants and mycorrhizal bodies on the individual and ramet, electrical propagation as a means of communication between plants has been hypothesized but understudied. This study aimed to test the hypothesis that plants can communicate with one another electrically via conductively isolated mycelial pathways. We created a bio-electric circuit linking two plants using a mycelial network grown from a blend of mycorrhizal fungi which was directly inoculated onto potato dextrose agar, or onto the host plants placed on the agar. The mycelium that grew was forced to cross, or "bridge," an air gap between the two islands of agar - thus forming the isolated conductive pathway between plants. Using this plant-fungal biocircuit we assessed electrical propagation between Pisum sativum and Cucumis sativus. We found that electrical signals were reliably conducted across the mycelial bridges from one plant to another upon the induction of a wound response. Our findings provide evidence that mechanical input can be communicated between plant species and opens the door to testing how this information can affect plant and fungal physiology.
Keywords: Plant; action; electric; electrophysiology; fungal; graded; mycelium; mycorrhizal; networks; potential; signaling.
Plain language summary
Most plants form underground relationships with fungi. These relationships are mutually beneficial. The plants and fungi share, trade, and distribute resources between themselves, their neighbors, and their offspring. Plants employ diverse methods to detect and respond to their environment and the production of electric signals is one of these methods. It would be favorable to a plant’s survival and the survival of their neighbors, if this plant could transmit and share the information these electrical signals contain. Possible avenues of transmission exist in the roots, and the fungi these roots are in contact with. If a fungal mass is in contact with the roots of multiple plants, it could propagate electrical signals throughout the plant network. We found that electric signals were reliably transmitted from one plant to another via fungal pathways upon the induction of a wound response. Our findings provide evidence that mechanical input can be communicated between plant species and opens the door to testing how this information can affect plant and fungal physiology.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







A patch of agar suitable for performing an agar touch on the side B.
A leaf suitable for a leaf nudge and leaf snip on Side B.
A leaf suitable for a leaf nudge and leaf snip on Side A.
Surgical wax is used as weights to hold down the roots under the paper towel, and to keep the roots in good contact with the agar they rest on.
Surgical wax used to secure the setup to the testing pad.
Glass electrode inserted into the plant stem on Side A.
Reference electrode adhered with silver paste to the taproot of the plant


References
-
- Chen Y, Zhao D-J, Wang Z-Y, Wang Z-Y, Tang G, Huang L.. Plant electrical signal classification based on waveform similarity. Algorithms. 2016;9:70. doi:10.3390/a9040070. - DOI
-
- Smith SE, Read DJ eds. Mycorrhizal symbiosis. 2nd ed. Cambridge, MA: Academic Press; 2016.
-
- Akhtar MS, Siddiqui Z. Arbuscular mycorrhizal fungi as potential bioprotectants against plant pathogens (Dordrecht, Nl: ). 2008. p. 6,61. doi:10.1007/978-1-4020-8770-7_3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
