Nematocyst sequestration within the family Fionidae (Gastropoda: Nudibranchia) considering ecological properties and evolution
- PMID: 36384570
- PMCID: PMC9670572
- DOI: 10.1186/s12983-022-00474-9
Nematocyst sequestration within the family Fionidae (Gastropoda: Nudibranchia) considering ecological properties and evolution
Abstract
Aeolid nudibranchs are well-known for their ability to incorporate cnidarian nematocysts and use them for defense; this process is tightly linked with the feeding preferences of molluscs. As many nudibranch groups show signs of ecology-based adaptive radiation, studies of prey-based defensive mechanisms can provide valuable insight into details of nudibranch evolutionary history. The main goal of this study is to test the correlation of ecological traits, feeding mechanisms, and prey preferences with cnidosac fine morphology and to pinpoint the phylogenetic value of these traits. We study the cnidosac morphology in thirteen species-representatives of the main lineages within the family Fionidae s.l. The morphological analysis includes histological sections, transmission electron microscopy, confocal laser scanning microscopy, and scanning electron microscopy. For phylogenetic study, available molecular data from public repositories were used, and phylogenetic trees were produced based on Bayesian Inference and Maximum likelihood analysis for a concatenated dataset of three molecular markers (COI, 16S, H3). In general, fionid cnidosacs fit the common aeolid pattern, but among different species we detected a high variation in type of obtained nematocysts, their arrangement within cnidophages, and in number of cell types within cnidosacs. We report on presence of cellules speciale in the haemocoel of all studied species, and for the first time, we report on cells with chitinous spindles in the haemocoel of all fionids except Eubranchus. The function of both these cell types remains unknown. The loss of functional cnidosacs occurred at least three times within Fionidae, and in case of the genera Phestilla, Calma, and Fiona, this loss is linked to their non-cnidarian diet. The diversity of cnidosac fine structure within Fionidae s.l. correlates with that of the radular morphology and feeding preferences of each species. Prey shifts between cnidarian and non-cnidarian prey (both through evolutionary shifts and individual variation) rarely occur within Fionidae s.l.; however, microevolutionary shifts between different hydrozoan species within a single genus are more common. Cnidosac morphology demonstrates considerable resulting changes even when switching between similar hydrozoan species, or changing the feeding site on same prey species. These data indicate that cnidosac morphology likely follows microevolutionary prey shifts-in other words, it is affected by switches in prey species and changes in feeding sites with a single prey species. Thus, the cnidosac morphology may be a useful indicator when studying ecological features of particular species.
Keywords: Adaptive radiation; Character evolution; Chitin; Feeding modes; Functional morphology; Kleptocnidae; Phylogeny.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they no competing interests.
Figures

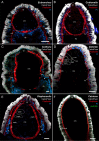
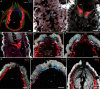
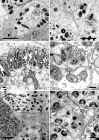

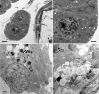

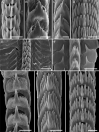

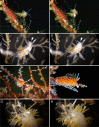

References
-
- Cimino G, Ghiselin MT. Chemical defense and evolutionary trends in biosynthetic capacity among dorid nudibranchs (Mollusca: Gastropoda: Opisthobranchia) Chemoecology. 1999;9(4):187–207.
-
- Wägele H. Potential key characters in Opisthobranchia (Gastropoda, Mollusca) enhancing adaptive radiation. Org Divers Evol. 2004;4(3):175–188.
-
- Burghardt I, Stemmer K, Wägele H. Symbiosis between Symbiodinium (Dinophyceae) and various taxa of Nudibranchia (Mollusca: Gastropoda), with analyses of long-term retention. Org Divers Evol. 2008;8(1):66–76.
-
- Burghardt I, Schrödl M, Wägele H. Three new solar-powered species of the genus Phyllodesmium Ehrenberg, 1831 (Mollusca: Nudibranchia: Aeolidioidea) from the tropical Indo-Pacific, with analysis of their photosynthetic activity and notes on biology. J Mollus Stud. 2008;74(3):277–292.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

