CBP-HSF2 structural and functional interplay in Rubinstein-Taybi neurodevelopmental disorder
- PMID: 36385105
- PMCID: PMC9668993
- DOI: 10.1038/s41467-022-34476-2
CBP-HSF2 structural and functional interplay in Rubinstein-Taybi neurodevelopmental disorder
Erratum in
-
Author Correction: CBP-HSF2 structural and functional interplay in Rubinstein-Taybi neurodevelopmental disorder.Nat Commun. 2023 Sep 28;14(1):6067. doi: 10.1038/s41467-023-41869-4. Nat Commun. 2023. PMID: 37770591 Free PMC article. No abstract available.
Abstract
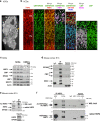
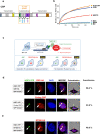
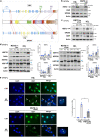
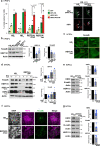
Patients carrying autosomal dominant mutations in the histone/lysine acetyl transferases CBP or EP300 develop a neurodevelopmental disorder: Rubinstein-Taybi syndrome (RSTS). The biological pathways underlying these neurodevelopmental defects remain elusive. Here, we unravel the contribution of a stress-responsive pathway to RSTS. We characterize the structural and functional interaction between CBP/EP300 and heat-shock factor 2 (HSF2), a tuner of brain cortical development and major player in prenatal stress responses in the neocortex: CBP/EP300 acetylates HSF2, leading to the stabilization of the HSF2 protein. Consequently, RSTS patient-derived primary cells show decreased levels of HSF2 and HSF2-dependent alteration in their repertoire of molecular chaperones and stress response. Moreover, we unravel a CBP/EP300-HSF2-N-cadherin cascade that is also active in neurodevelopmental contexts, and show that its deregulation disturbs neuroepithelial integrity in 2D and 3D organoid models of cerebral development, generated from RSTS patient-derived iPSC cells, providing a molecular reading key for this complex pathology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

