Fragment-based virtual screening identifies a first-in-class preclinical drug candidate for Huntington's disease
- PMID: 36385140
- PMCID: PMC9668931
- DOI: 10.1038/s41598-022-21900-2
Fragment-based virtual screening identifies a first-in-class preclinical drug candidate for Huntington's disease
Abstract
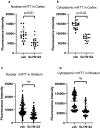
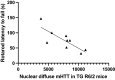
Currently, there are no therapies available to modify the disease progression of Huntington's disease (HD). Recent clinical trial failures of antisense oligonucleotide candidates in HD have demonstrated the need for new therapeutic approaches. Here, we developed a novel in-silico fragment scanning approach across the surface of mutant huntingtin (mHTT) polyQ and predicted four hit compounds. Two rounds of compound analoging using a strategy of testing structurally similar compounds in an affinity assay rapidly identified GLYN122. In vitro, GLYN122 directly binds and reduces mHTT and induces autophagy in neurons. In vivo, our results confirm that GLYN122 can reduce mHTT in the cortex and striatum of the R/2 mouse model of Huntington's disease and subsequently improve motor symptoms. Thus, the in-vivo pharmacology profile of GLYN122 is a potential new preclinical candidate for the treatment of HD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. SME is the Chief Executive Officer (CEO) of Galyan Bio, Inc. CYE is a professor at ETH Zurich but also a co-founder and shareholder of Avea Life AG, and is on the Scientific Advisory Board of Maximon AG and Galyan Bio, Inc. KL is Managing Director of Bicoll GmbH and Director and General Manager of Bicoll Biotechnology (Shanghai) Co. Ltd. All other authors do not have any conflicts of interest to declare.
Figures
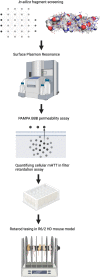
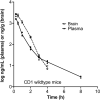
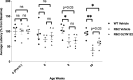

Similar articles
-
Pramipexole reduces soluble mutant huntingtin and protects striatal neurons through dopamine D3 receptors in a genetic model of Huntington's disease.Exp Neurol. 2018 Jan;299(Pt A):137-147. doi: 10.1016/j.expneurol.2017.10.019. Epub 2017 Oct 19. Exp Neurol. 2018. PMID: 29056363
-
Genetic manipulations of mutant huntingtin in mice: new insights into Huntington's disease pathogenesis.FEBS J. 2013 Sep;280(18):4382-94. doi: 10.1111/febs.12418. Epub 2013 Jul 31. FEBS J. 2013. PMID: 23829302 Free PMC article. Review.
-
LAMP2A-mediated autophagy involved in Huntington's disease progression.Biochem Biophys Res Commun. 2021 Jan 1;534:561-567. doi: 10.1016/j.bbrc.2020.11.042. Epub 2020 Nov 22. Biochem Biophys Res Commun. 2021. PMID: 33239172
-
Effects of Exogenous NUB1 Expression in the Striatum of HDQ175/Q7 Mice.J Huntingtons Dis. 2016 Jun 13;5(2):163-74. doi: 10.3233/JHD-160195. J Huntingtons Dis. 2016. PMID: 27314618
-
Nature and cause of mitochondrial dysfunction in Huntington's disease: focusing on huntingtin and the striatum.J Neurochem. 2010 Jul;114(1):1-12. doi: 10.1111/j.1471-4159.2010.06741.x. Epub 2010 Apr 9. J Neurochem. 2010. PMID: 20403078 Review.
Cited by
-
Huntington's Disease Drug Development: A Phase 3 Pipeline Analysis.Pharmaceuticals (Basel). 2023 Oct 24;16(11):1513. doi: 10.3390/ph16111513. Pharmaceuticals (Basel). 2023. PMID: 38004378 Free PMC article. Review.
-
Longevity biotechnology: bridging AI, biomarkers, geroscience and clinical applications for healthy longevity.Aging (Albany NY). 2024 Oct 16;16(20):12955-12976. doi: 10.18632/aging.206135. Epub 2024 Oct 16. Aging (Albany NY). 2024. PMID: 39418098 Free PMC article. Review.
-
Correlation of protein binding pocket properties with hits' chemistries used in generation of ultra-large virtual libraries.J Comput Aided Mol Des. 2024 May 16;38(1):22. doi: 10.1007/s10822-024-00562-4. J Comput Aided Mol Des. 2024. PMID: 38753096 Free PMC article.
-
HD_BPMDS: a curated binary pattern multitarget dataset of Huntington's disease-targeting agents.J Cheminform. 2023 Nov 17;15(1):109. doi: 10.1186/s13321-023-00775-z. J Cheminform. 2023. PMID: 37978560 Free PMC article.
-
Emerging Therapies for Huntington's Disease - Focus on N-Terminal Huntingtin and Huntingtin Exon 1.Biologics. 2022 Sep 30;16:141-160. doi: 10.2147/BTT.S270657. eCollection 2022. Biologics. 2022. PMID: 36213816 Free PMC article. Review.
References
-
- Bates GP, et al. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. - PubMed
-
- Caron, N. S., Wright, G. E. & Hayden, M. R. GeneReviews: Huntington disease. Gene Rev.https://www.ncbi.nlm.nih.gov/books/NBK1305/ (2018).
-
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

