Ferroptosis inhibition by deferiprone, attenuates myelin damage and promotes neuroprotection in demyelinated optic nerve
- PMID: 36385152
- PMCID: PMC9668997
- DOI: 10.1038/s41598-022-24152-2
Ferroptosis inhibition by deferiprone, attenuates myelin damage and promotes neuroprotection in demyelinated optic nerve
Abstract
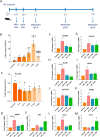
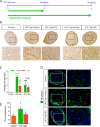
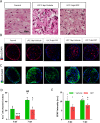
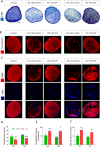
Multiple sclerosis (MS) is a chronic inflammatory disease, which leads to focal demyelination in the brain and spinal cord. Studies showed that iron released during the course of myelin breakdown exacerbates tissue damage, which is in agreement with the features of iron-dependent cell death, ferroptosis. Here, we aimed to investigate the possible contribution of ferroptosis in the demyelinated optic nerve, and to explore the effectiveness of ferroptosis inhibitor, deferiprone (DFP), on the extent of demyelination, inflammation and axonal damage. For this purpose, focal demyelination was induced by injection of lysolecithin (LPC), into the optic nerve of male C57BL/6J mice. Afterward, optic nerves were harvested at different time points from as early as 6 h up to 7 days post-LPC injection. Next, to evaluate the effectiveness of DFP two groups of animals received daily intraperitoneal injection of DFP for 3 or 7 continuous days. Vehicle groups received saline. Iron deposition was observed at different time points post-LPC injection from 6 h to 7 days post injection. Examining ferroptosis markers showed a significant reduction in glutathione content along with increased level of malondialdehyde and upregulated ferroptosis marker genes at early time points after injection. Besides, DFP treatment during the inflammatory phase of the model resulted in decreased microgliosis and inflammation. Reduced demyelination, microgliosis and astrogliosis was shown in mice that received DFP for 7 days. Moreover, DFP protected against axonal damage and retinal ganglion cells loss. Our results suggest the possible contribution of ferroptosis pathway in the process of demyelination. The therapeutic strategies targeting iron deposition, e.g. DFP treatment might thus represent a promising therapeutic target for patients with MS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Lassmann H, Van Horssen J, Mahad D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012;8:647–656. - PubMed
-
- Möller HE, et al. Iron, myelin, and the brain: Neuroimaging meets neurobiology. Trends Neurosci. 2019;42:384–401. - PubMed
-
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: The role of iron. Glia. 2009;57:467–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

