Open-channel structure of a pentameric ligand-gated ion channel reveals a mechanism of leaflet-specific phospholipid modulation
- PMID: 36385237
- PMCID: PMC9668969
- DOI: 10.1038/s41467-022-34813-5
Open-channel structure of a pentameric ligand-gated ion channel reveals a mechanism of leaflet-specific phospholipid modulation
Abstract
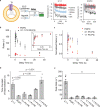
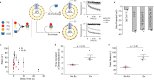
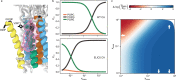
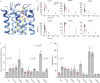
Pentameric ligand-gated ion channels (pLGICs) mediate synaptic transmission and are sensitive to their lipid environment. The mechanism of phospholipid modulation of any pLGIC is not well understood. We demonstrate that the model pLGIC, ELIC (Erwinia ligand-gated ion channel), is positively modulated by the anionic phospholipid, phosphatidylglycerol, from the outer leaflet of the membrane. To explore the mechanism of phosphatidylglycerol modulation, we determine a structure of ELIC in an open-channel conformation. The structure shows a bound phospholipid in an outer leaflet site, and structural changes in the phospholipid binding site unique to the open-channel. In combination with streamlined alchemical free energy perturbation calculations and functional measurements in asymmetric liposomes, the data support a mechanism by which an anionic phospholipid stabilizes the activated, open-channel state of a pLGIC by specific, state-dependent binding to this site.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Direct binding of phosphatidylglycerol at specific sites modulates desensitization of a ligand-gated ion channel.Elife. 2019 Nov 14;8:e50766. doi: 10.7554/eLife.50766. Elife. 2019. PMID: 31724949 Free PMC article.
-
Lipid nanodisc scaffold and size alter the structure of a pentameric ligand-gated ion channel.Nat Commun. 2024 Jan 2;15(1):25. doi: 10.1038/s41467-023-44366-w. Nat Commun. 2024. PMID: 38167383 Free PMC article.
-
Cryo-EM structures of a pentameric ligand-gated ion channel in liposomes.Elife. 2025 Jul 16;14:RP106728. doi: 10.7554/eLife.106728. Elife. 2025. PMID: 40668221 Free PMC article.
-
Structural basis for the modulation of pentameric ligand-gated ion channel function by lipids.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183304. doi: 10.1016/j.bbamem.2020.183304. Epub 2020 Apr 18. Biochim Biophys Acta Biomembr. 2020. PMID: 32311340 Review.
-
Recent Insight into Lipid Binding and Lipid Modulation of Pentameric Ligand-Gated Ion Channels.Biomolecules. 2022 Jun 10;12(6):814. doi: 10.3390/biom12060814. Biomolecules. 2022. PMID: 35740939 Free PMC article. Review.
Cited by
-
State-dependent binding of cholesterol and an anionic lipid to the muscle-type Torpedo nicotinic acetylcholine receptor.Commun Biol. 2024 Apr 10;7(1):437. doi: 10.1038/s42003-024-06106-8. Commun Biol. 2024. PMID: 38600247 Free PMC article.
-
Sterol derivative binding to the orthosteric site causes conformational changes in an invertebrate Cys-loop receptor.Elife. 2023 Jul 3;12:e86029. doi: 10.7554/eLife.86029. Elife. 2023. PMID: 37395731 Free PMC article.
-
A Guide to Your Desired Lipid-Asymmetric Vesicles.Membranes (Basel). 2023 Feb 23;13(3):267. doi: 10.3390/membranes13030267. Membranes (Basel). 2023. PMID: 36984654 Free PMC article. Review.
-
Structural studies of the human α1 glycine receptor via site-specific chemical cross-linking coupled with mass spectrometry.Biophys Rep (N Y). 2024 Dec 11;4(4):100184. doi: 10.1016/j.bpr.2024.100184. Epub 2024 Oct 10. Biophys Rep (N Y). 2024. PMID: 39393591 Free PMC article.
-
Mechanisms underlying modulation of human GlyRα3 by Zn2+ and pH.Sci Adv. 2024 Dec 20;10(51):eadr5920. doi: 10.1126/sciadv.adr5920. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693447 Free PMC article.
References
-
- Corringer P-J, et al. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure. 2012;20:941–956. - PubMed
-
- Bäckström T, et al. Allopregnanolone and mood disorders. Prog. Neurobiol. 2014;113:88–94. - PubMed
-
- Meltzer-Brody S, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058–1070. - PubMed
-
- Gunduz-Bruce H, et al. Trial of SAGE-217 in patients with major depressive disorder. N. Engl. J. Med. 2019;381:903–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

