Multisensory integration of orally-sourced gustatory and olfactory inputs to the posterior piriform cortex in awake rats
- PMID: 36385245
- PMCID: PMC9869978
- DOI: 10.1113/JP283873
Multisensory integration of orally-sourced gustatory and olfactory inputs to the posterior piriform cortex in awake rats
Abstract
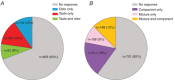
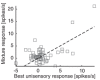
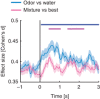
Flavour refers to the sensory experience of food, which is a combination of sensory inputs sourced from multiple modalities during consumption, including taste and odour. Previous work has demonstrated that orally-sourced taste and odour cues interact to determine perceptual judgements of flavour stimuli, although the underlying cellular- and circuit-level neural mechanisms remain unknown. We recently identified a region of the piriform olfactory cortex in rats that responds to both taste and odour stimuli. Here, we investigated how converging taste and odour inputs to this area interact to affect single neuron responsiveness ensemble coding of flavour identity. To accomplish this, we recorded spiking activity from ensembles of single neurons in the posterior piriform cortex (pPC) in awake, tasting rats while delivering taste solutions, odour solutions and taste + odour mixtures directly into the oral cavity. Our results show that taste and odour inputs evoke highly selective, temporally-overlapping responses in multisensory pPC neurons. Comparing responses to mixtures and their unisensory components revealed that taste and odour inputs interact in a non-linear manner to produce unique response patterns. Taste input enhances trial-by-trial decoding of odour identity from small ensembles of simultaneously recorded neurons. Together, these results demonstrate that taste and odour inputs to pPC interact in complex, non-linear ways to form amodal flavour representations that enhance identity coding. KEY POINTS: Experience of food involves taste and smell, although how information from these different senses is combined by the brain to create our sense of flavour remains unknown. We recorded from small groups of neurons in the olfactory cortex of awake rats while they consumed taste solutions, odour solutions and taste + odour mixtures. Taste and smell solutions evoke highly selective responses. When presented in a mixture, taste and smell inputs interacted to alter responses, resulting in activation of unique sets of neurons that could not be predicted by the component responses. Synergistic interactions increase discriminability of odour representations. The olfactory cortex uses taste and smell to create new information representing multisensory flavour identity.
Keywords: cross-modal; decoding; flavour; odour; olfactory cortex; retronasal; taste.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Taste-Odor Association Learning Alters the Dynamics of Intraoral Odor Responses in the Posterior Piriform Cortex of Awake Rats.eNeuro. 2023 Mar 29;10(3):ENEURO.0010-23.2023. doi: 10.1523/ENEURO.0010-23.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36898831 Free PMC article.
-
Gustatory cortex neurons perform reliability-dependent integration of multisensory flavor inputs.Curr Biol. 2025 Feb 3;35(3):600-611.e3. doi: 10.1016/j.cub.2024.12.015. Epub 2025 Jan 10. Curr Biol. 2025. PMID: 39798562
-
Single-neuron responses to intraoral delivery of odor solutions in primary olfactory and gustatory cortex.J Neurophysiol. 2017 Mar 1;117(3):1293-1304. doi: 10.1152/jn.00802.2016. Epub 2016 Dec 21. J Neurophysiol. 2017. PMID: 28003413 Free PMC article.
-
Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion.Acta Physiol Hung. 2008 Jun;95(2):131-64. doi: 10.1556/APhysiol.95.2008.2.1. Acta Physiol Hung. 2008. PMID: 18642756 Review.
-
The rules of formation of the olfactory representations found in the orbitofrontal cortex olfactory areas in primates.Chem Senses. 2001 Jun;26(5):595-604. doi: 10.1093/chemse/26.5.595. Chem Senses. 2001. PMID: 11418505 Review.
Cited by
-
Neural Processing of Taste-Related Signals in the Mediodorsal Thalamus of Mice.bioRxiv [Preprint]. 2025 Feb 17:2024.08.05.606609. doi: 10.1101/2024.08.05.606609. bioRxiv. 2025. Update in: J Neurosci. 2025 Mar 26:e1500242025. doi: 10.1523/JNEUROSCI.1500-24.2025. PMID: 39149395 Free PMC article. Updated. Preprint.
-
Taste enhances the ability to express a preference for a congruent odor in rats.Behav Neurosci. 2024 Dec;138(6):433-440. doi: 10.1037/bne0000605. Epub 2024 Sep 19. Behav Neurosci. 2024. PMID: 39298234 Free PMC article.
-
Taste-Odor Association Learning Alters the Dynamics of Intraoral Odor Responses in the Posterior Piriform Cortex of Awake Rats.eNeuro. 2023 Mar 29;10(3):ENEURO.0010-23.2023. doi: 10.1523/ENEURO.0010-23.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36898831 Free PMC article.
-
Gustatory cortex neurons perform reliability-dependent integration of multisensory flavor inputs.Curr Biol. 2025 Feb 3;35(3):600-611.e3. doi: 10.1016/j.cub.2024.12.015. Epub 2025 Jan 10. Curr Biol. 2025. PMID: 39798562
-
Multisensory Integration Underlies the Distinct Representation of Odor-Taste Mixtures in the Gustatory Cortex of Behaving Rats.J Neurosci. 2024 May 15;44(20):e0071242024. doi: 10.1523/JNEUROSCI.0071-24.2024. J Neurosci. 2024. PMID: 38548337 Free PMC article.
References
-
- Auvray, M. , & Spence, C. (2008). The multisensory perception of flavor. Consciousness and Cognition, 17(3), 1016–1031. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

