Human T lymphocytes at tumor sites
- PMID: 36385379
- PMCID: PMC9668216
- DOI: 10.1007/s00281-022-00970-4
Human T lymphocytes at tumor sites
Abstract
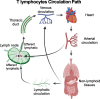
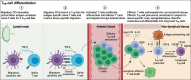
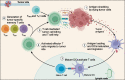
CD4+ and CD8+ T lymphocytes mediate most of the adaptive immune response against tumors. Naïve T lymphocytes specific for tumor antigens are primed in lymph nodes by dendritic cells. Upon activation, antigen-specific T cells proliferate and differentiate into effector cells that migrate out of peripheral blood into tumor sites in an attempt to eliminate cancer cells. After accomplishing their function, most effector T cells die in the tissue, while a small fraction of antigen-specific T cells persist as long-lived memory cells, circulating between peripheral blood and lymphoid tissues, to generate enhanced immune responses when re-encountering the same antigen. A subset of memory T cells, called resident memory T (TRM) cells, stably resides in non-lymphoid peripheral tissues and may provide rapid immunity independently of T cells recruited from blood. Being adapted to the tissue microenvironment, TRM cells are potentially endowed with the best features to protect against the reemergence of cancer cells. However, when tumors give clinical manifestation, it means that tumor cells have evaded immune surveillance, including that of TRM cells. Here, we review the current knowledge as to how TRM cells are generated during an immune response and then maintained in non-lymphoid tissues. We then focus on what is known about the role of CD4+ and CD8+ TRM cells in antitumor immunity and their possible contribution to the efficacy of immunotherapy. Finally, we highlight some open questions in the field and discuss how new technologies may help in addressing them.
Keywords: Cancer immunology; Immunotherapy; Memory T cell differentiation; T cell homing; Tissue-resident T cells; Trm cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

